Rationale and Objectives
Ultrasound-targeted microbubble destruction is a promising technology for the targeted gene delivery. The purpose of the present study is to prepare a novel lipid ultrasound microbubble-carrying gene and transactivating transcriptional activator (Tat) peptide and to investigate its transfection effect in vivo.
Methods and Materials
Lipid ultrasound microbubbles were prepared using mechanical vibration, and the appearance, distribution, concentration, diameter, and zeta potential of the lipid ultrasound microbubbles were measured. The efficiencies of the microbubble carrying gene and Tat peptide were investigated using the fluorospectrophotometer. Contrast-enhanced ultrasonography was performed on normal rabbits to observe the duration and intensity of enhancement in myocardium. Quantitative analysis was detected using the DFY Ultrasound Image Analyzer. Transfection in vivo was performed using the CGZZ ultrasound gene transfection instrument. The expression of enhanced green fluorescent protein in the organs was observed using confocal laser scanning microscope.
Results
The diameter of the lipid microbubbles carrying gene and Tat was (2.3 ± 0.4) μm, the concentration was (3.1 ± 0.4) ×10 9 /mL, and Zeta potential was (2.0 ± 0.1) mV. The gene encapsulation efficiency of the lipid ultrasound microbubbles was 32%, and the Tat encapsulation efficiency was 35%. In vivo experiment showed that lipid ultrasound microbubbles could enhance the echo intensity and transfection efficiency.
Conclusion
Lipid microbubbles containing gene and Tat peptide can be used as a new vehicle for gene transfection.
Gene therapy shows considerable promise as a novel treatment modality for human diseases . Gene delivery technique includes viral vector and nonviral vector, but the technique is limited by low safety of viral vector and poor efficiency of nonviral vector. As a negatively charged macromolecule, DNA can not enter cells actively and is rapidly degraded by nucleases . Therefore, developing an efficient gene delivery system is necessary.
Besides the well-known application of microbubbles as contrast agents for diagnostic ultrasound (US), microbubbles have also been demonstrated to be an effective technique for targeted gene delivery . Several studies investigated the mechanisms for US-targeted microbubble destruction (UTMD) enhancing gene delivery into cells. Electron microscopy demonstrated pore formation on cell membranes immediately after destruction of microbubbles. This phenomenon was transient and the pores disappeared after 24 hours. Such “sonoporation” effects may facilitate gene to entry into the cell. It was then postulated that these transient holes in the cell surface caused by UTMD resulted in a rapid translocation of gene from outside to cytoplasm . Christiansen et al showed that plasmid DNA remained intact after being loaded onto and subsequently released from microbubbles by US irradiation, and confirmed that UTMD was a novel and highly efficient gene delivery carrier. The technique involves intravenous or intraarterial injection of echogenic microbubbles combining with genetic materials, and then US is used to destroy microbubbles in the targeted tissue. Use of UTMD provides many desirable characteristics for gene therapy, including low toxicity, low immunogenicity, low invasiveness, the potential for repeated application, organ specificity, and broad applicability to sonographically accessible targets. However, gene material released from microbubbles destructed by US radiation only passively enters into targeted cells; therefore, the transfection efficiency is still limited.
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Materials and methods
Plasmid DNA
Get Radiology Tree app to read full this article<
Tat Peptide
Get Radiology Tree app to read full this article<
Cell Culture and Transfection
Get Radiology Tree app to read full this article<
Preparation of Microbubbles
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Characterization of the Microbubbles
Get Radiology Tree app to read full this article<
Confocal Laser Scanning Microscopy
Get Radiology Tree app to read full this article<
Encapsulation Efficiency of Plasmid DNA and Tat Peptide in Microbubbles
Get Radiology Tree app to read full this article<
Contrast-enhanced Imaging in vivo
Get Radiology Tree app to read full this article<
Quantitative Analysis
Get Radiology Tree app to read full this article<
Transfection in vivo
Get Radiology Tree app to read full this article<
Statistical Analysis
Get Radiology Tree app to read full this article<
Results
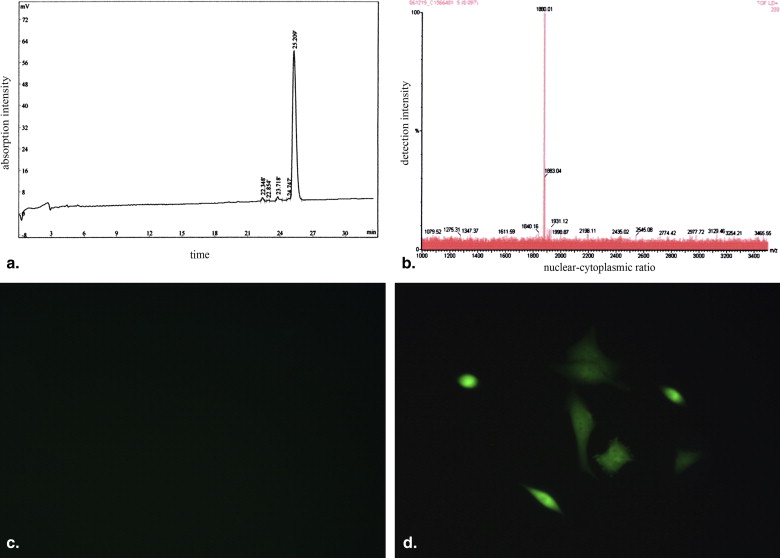
Tat Peptide Synthesis and Bioactivity Assay
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Physical Characterization of Microbubbles
Get Radiology Tree app to read full this article<
Table 1
Physical Characteristics of Lipid Ultrasound Contrast Agent (X¯¯¯±s) (
X
¯
±
s
)
Group Diameter (μm) Electric potential (mV) Concentration (×10 9 /mL) Blanked microbubbles 2.2 ± 0.7 −15.7 ± 0.9 3.3 ± 0.3 Microbubbles carrying gene 2.2 ± 0.7 −18.4 ± 2.0 2.9 ± 0.3 Microbubbles carrying
gene and Tat 2.3 ± 0.4 +2.0 ± 0.1 ∗ 3.1 ± 0.4
Compared with the blanked microbubbles.
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
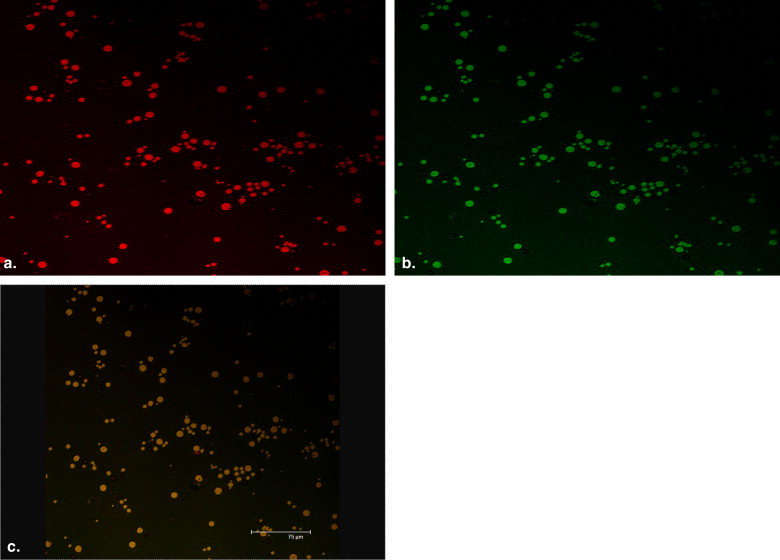
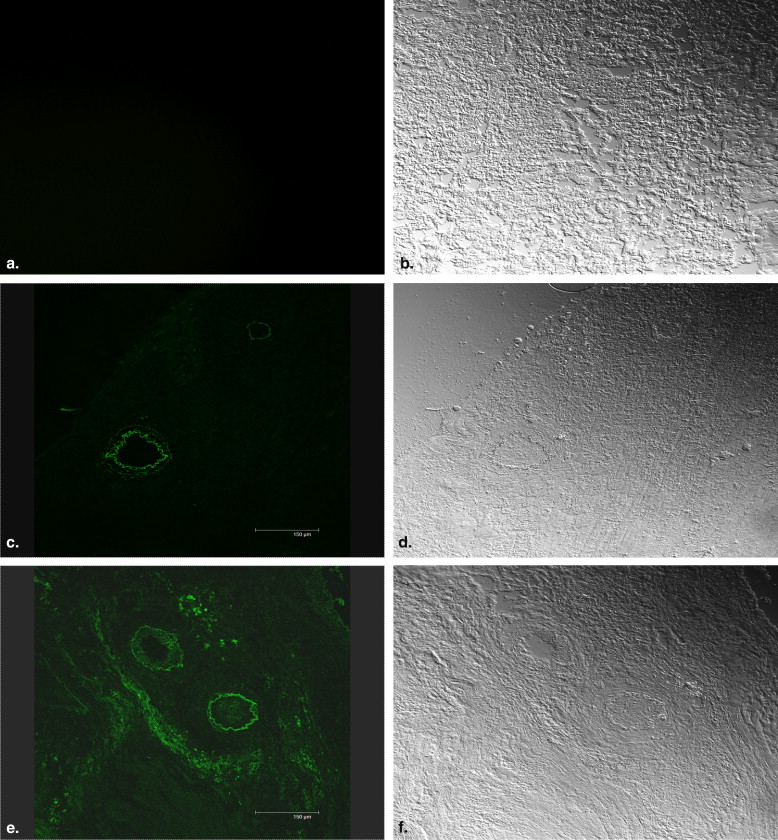
Confocal Laser Scanning Microscopy
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
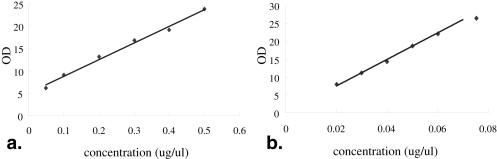
Encapsulation Efficiencies of Plasmid DNA and Tat Peptide in Microbubbles
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
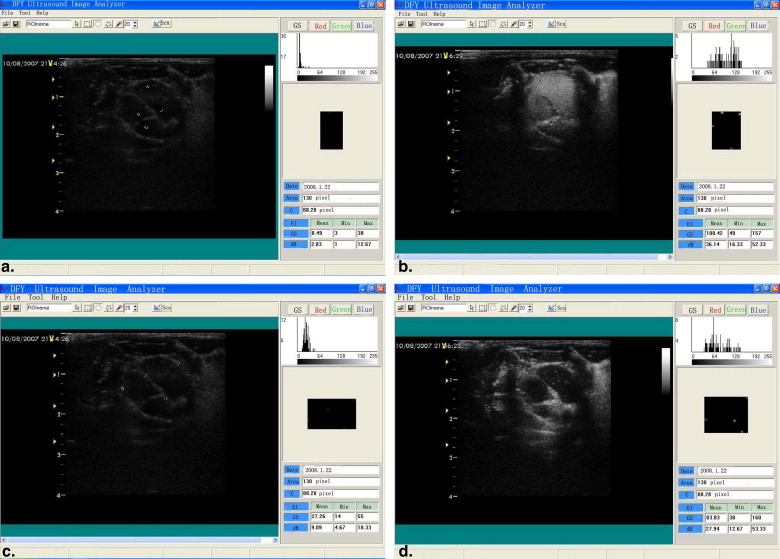
Heart Contrast Imaging In Vivo and Quantitative Analysis
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Transfection in vivo
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Discussion
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
References
1. Lyon A.R., Sato M., Hajjar R.J., et. al.: Gene therapy: targeting the myocardium. Heart 2008; 94: pp. 89-99.
2. Rossi J.J., June C.H., Kohn D.B.: Genetic therapies against HIV. Nat Biotechnol 2007; 25: pp. 1444-1454.
3. Chang C.W., Christensen L.V., Lee M., et. al.: Efficient expression of vascular endothelial growth factor using minicircle DNA for angiogenic gene therapy. J Control Release 2008; 125: pp. 155-163.
4. Patil S.D., Rhodes D.G., Burgess D.J.: DNA-based therapeutics and DNA delivery systems: a comprehensive review. AAPS J 2005; 7: pp. E61-E77.
5. Newman C.M., Bettinger T.: Gene therapy progress and prospects: ultrasound for gene transfer. Gene Ther 2007; 14: pp. 465-475.
6. Mehier-Humbert S., Yan F., Frinking P., et. al.: Ultrasound-mediated gene delivery: influence of contrast agent on transfection. Bioconjug Chem 2007; 18: pp. 652-662.
7. Suzuki R., Takizawa T., Negishi Y., et. al.: Effective gene delivery with liposomal bubbles and ultrasound as novel non-viral system. J Drug Target 2007; 15: pp. 531-537.
8. Bekeredjian R., Chen S., Frenkel P.A., et. al.: Ultrasound-targeted microbubble destruction can repeatedly direct highly specific plasmid expression to the heart. Circulation 2003; 108: pp. 1022-1026.
9. Bao S., Thrall B.D., Miller D.L.: Transfection of a reporter plasmid into cultured cells by sonoporation in vitro. Ultrasound Med Biol 1997; 23: pp. 953-957.
10. Miller D.L., Quddus J.: Sonoporation of monolayer cells by diagnostic ultrasound activation of contrast agent gas bodies. Ultrasound Med Biol 2000; 26: pp. 661-667.
11. Miller D.L., Pislaru S.V., Greenleaf J.E.: Sonoporation: mechanical DNA delivery by ultrasonic cavitation. Somat Cell Mol Genet 2002; 27: pp. 115-134.
12. Christiansen J.P., French B.A., Klibanov A.L., et. al.: Targeted tissue transfection with ultrasound destruction of plasmid-bearing cationic microbubbles. Ultrasound Med Biol 2003; 29: pp. 1759-1767.
13. Torchilin V.P., Levchenko T.S., Rammohan R., et. al.: Cell transfection in vitro and in vivo with nontoxic TAT peptide-liposome-DNA complexes. Proc Natl Acad Sci U S A 2003; 100: pp. 1972-1977.
14. Liu Z., Li M., Cui D., et. al.: Macro-branched cell-penetrating peptide design for gene delivery. J Control Release 2005; 102: pp. 699-710.
15. Saleh A.F., Aojula H.S., Pluen A.: Enhancement of gene transfer using YIGSR analog of Tat-derived peptide. Biopolymers 2008; 89: pp. 62-71.
16. Vives E., Brodin P., Lebleu B.: A truncated HIV-1 Tat protein basic domain rapidly translocates through the plasma membrane and accumulates in the cell nucleus. J Biol Chem 1997; 272: pp. 16010-16017.
17. Xingsheng L., Zhigang W., Lin S., et. al.: Construction and expression of enhanced green fluorescent protein and HGF co-expression vector. J Chongqing Med Univ 2007; 32: pp. 470-473.
18. Shohet R.V., Chen S., Zhou Y.T., et. al.: Echocardiographic destruction of albumin microbubbles directs gene delivery to the myocardium. Circulation 2000; 101: pp. 2554-2556.
19. Frenkel P.A., Chen S., Thai T., et. al.: DNA-loaded albumin microbubbles enhance ultrasound-mediated transfection in vitro. Ultrasound Med Biol 2002; 28: pp. 817-822.
20. Erikson J.M., Freeman G.L., Chandrasekar B.: Ultrasound-targeted antisense oligonucleotide attenuates ischemia/reperfusion-induced myocardial tumor necrosis factor-alpha. J Mol Cell Cardiol 2003; 35: pp. 119-130.
21. Unger E.C., Matsunaga T.O., McCreery T., et. al.: Therapeutic applications of microbubbles. Eur J Radiol 2002; 4: pp. 160-168.
22. Lawrie A., Brisken A.F., Francis S.E., et. al.: Microbubble-enhanced ultrasound for vascular gene delivery. Gene Ther 2000; 7: pp. 2023-2027.
23. Pislaru S.V., Pislaru C., Kinnick R.R., et. al.: Optimization of ultrasound mediated gene transfer: comparison of contrast agents and ultrasound modalities. Eur Heart J 2003; 24: pp. 1690-1698.
24. Mukherjee D., Wong J., Griffin B., et. al.: Ten-fold augmentation of endothelial uptake of vascular endothelial growth factor with ultra-sound after systemic administration. J Am Coll Cardiol 2000; 35: pp. 1678-1686.
25. Price R.J., Skyba D.M., Kaul S., et. al.: Delivery of colloidal particles and red blood cells to tissue through microvessel ruptures created by targeted microbubble destruction with ultrasound. Circulation 1998; 98: pp. 1264-1267.
26. Song J., Chappell J.C., Qi M., et. al.: Influence of injection site, microvascular pressure and ultrasound variables on microbubble-mediated delivery of microspheres to muscle. J Am Coll Cardiol 2002; 39: pp. 726-731.
27. Kondo I., Ohmori K., Oshita A., et. al.: Treatment of acute myocardial infarction by hepatocyte growth factor gene transfer. J Am Coll Cardiol 2004; 44: pp. 644-653.
28. Masaki T., Norio H., Toshihisa Y.: Effect of oligopeptides on gene expression: comparison of DNA/peptide and DNA/peptide/liposome complexes. Int J Pharm 2004; 269: pp. 71-80.
29. Hyndman L., Lemoine J.L., Huang L., et. al.: HIV-1 Tat protein transduction domain peptide facilitates gene transfer in combination with cationic liposomes. Control Release 2004; 99: pp. 435-444.
30. Ziegler A., Seelig J.: High affinity of the cell-penetrating peptide HIV-1 Tat-PTD for DNA. Biochemistry 2007; 46: pp. 8138-8145.
31. Siprashvili Z., Scholl F.A., Oliver S.F., et. al.: Gene transfer via reversible plasmid condensation with cysteine-flanked, internally spaced arginine-rich peptides. Hum Gene Ther 2003; 14: pp. 1225-1233.