Rationale and Objectives
To perform a meta-analysis to assess the diagnostic performance of the diffusion-weighted magnetic resonance imaging (DWI) technique in discrimination of benign and malignant pulmonary nodules or masses.
Materials and Methods
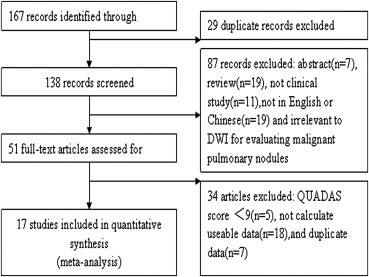
Data sources were studies published in PubMed, MEDLINE, EMBASE, Cochrane Library, and China National Knowledge Infrastructure databases from January 2001 to May 2013. Studies evaluating the diagnostic accuracy of DWI for benign/malignant discrimination of pulmonary nodules in English or Chinese language were considered for inclusion. Methodological quality was assessed by the quality assessment of diagnostic studies instrument. Sensitivities, specificities, predictive values, diagnostic odds ratios (DORs), and areas under the receiver operating characteristic curve (AUCs) were calculated. Potential threshold effect, heterogeneity, and publication bias were investigated. We also evaluated the clinical utility of DWI in diagnosis of lung lesions.
Results
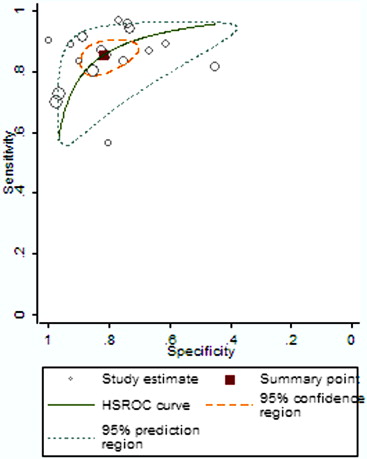
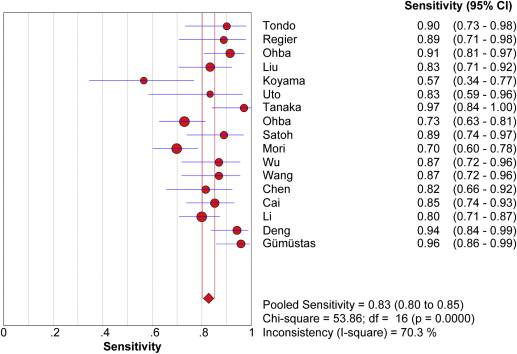
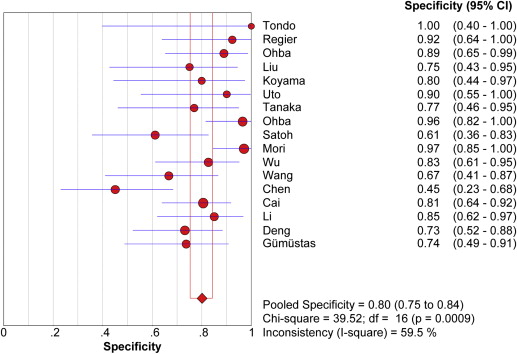
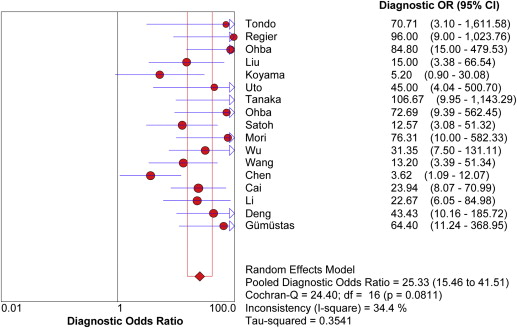
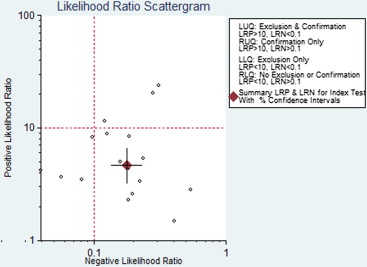
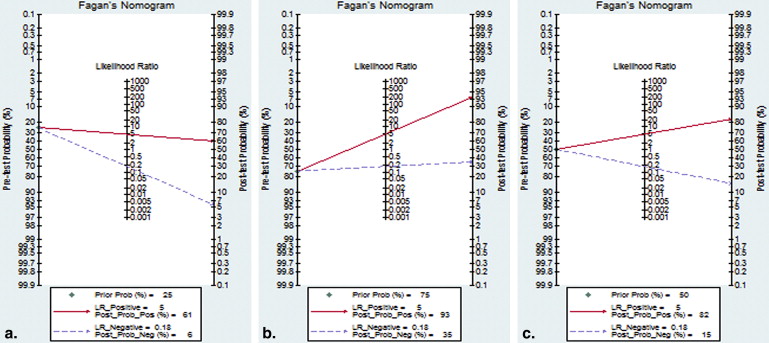
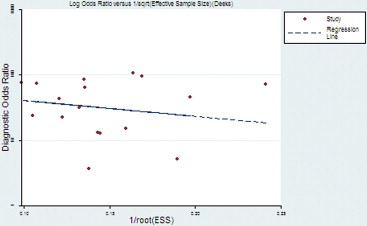
Seventeen studies comprising 855 malignant and 322 benign lesions were included in this meta-analysis. There was no significant threshold effect. Summary receiver operating characteristic curve showed that AUC was 0.909 (95% confidence interval [CI], 0.862–0.931). Pooled weighted estimates of sensitivity, specificity, positive likelihood ratio (PLR), and negative likelihood ratio (NLR) were 0.828 (95% CI, 0.801–0.853), 0.801 (95% CI, 0.753–0.843), 4.01 (95% CI, 2.78–5.80), and 0.20 (95% CI, 0.15–0.27), respectively. Heterogeneity was found to have stemmed primarily from study design (retrospective or prospective study). Subgroup analysis showed that diagnostic performance (sensitivity, 0.88; 95% CI, 0.82–0.92 and specificity, 0.89; 95% CI, 0.79–0.96) of retrospectively designed studies was significantly higher than that of prospectively designed studies. The Deeks’ funnel plot indicated the absence of publication bias.
Conclusions
With respect to the accuracy and DOR, DWI is useful for differentiation between malignant and benign pulmonary nodules or masses. Diagnostic test accuracy is not the be-all and end-all of diagnostic testing. Concerning PLR and NLR, DWI may not help to alter posttest probability compared to pretest probability to sufficiently alter physician’s decision making. Future analyses should be conducted in large-scale, high-quality trials to evaluate its clinical value and establish standards of DWI measurement, analysis, and cutoff values of diagnosis.
Pulmonary malignant lesion, especially lung cancer, is one of the leading causes of death . It usually presents as a solid nodule or mass on chest radiography or computed tomography (CT). Nowadays, there are mainly two common noninvasive methods for examining pulmonary nodules: positron emission tomography (PET) and CT . However, PET has been known to give false-positive results in inflammatory nodules or masses and false-negative results in well-differentiated pulmonary adenocarcinoma . In addition, high cost of fluorodeoxyglucose-PET restricts its clinical application . Although many well-known characteristics based on shape, size, and internal characteristics have been described and proven to be useful for lesion differentiation on contrast-enhanced CT, it remains a challenge for radiologists and clinicians to differentiate malignant and benign lesions on CT because there are some overlaps especially between hypervascular benign tumors or active granulomas and malignant nodules . Moreover, both PET and CT are radioactive. For these reasons, a nonradioactive and accurate alternative method is still desirable.
Recently, diffusion-weighted magnetic resonance imaging (DWI) has been promoted as an advantageous method, which is applied to various body parts for clinical use. Advantages of DWI lie in neither delivering radiation doses nor requiring exogenous contrast medium. As a new type of magnetic resonance imaging (MRI) functional imaging, it can provide quantitative and qualitative information about the integrity of cell membranes and tissue consistency. In the past, lung/chest imaging was the contraindication of MRI. Nevertheless, recent advances in fast imaging techniques such as echo planar imaging and parallel imaging make MRI more suitable for lung/chest imaging . Many studies have explored the role of DWI in differentiation of malignant and benign lesions, but there are some inconclusive or conflicting results published . Our study objective was to perform a meta-analysis to derive a more comprehensive and precise assessment for the overall diagnostic accuracy of DWI in the diagnosis of malignant pulmonary nodules or masses.
Methods
Publication Search
Get Radiology Tree app to read full this article<
Inclusion and Exclusion Criteria
Get Radiology Tree app to read full this article<
Data Extraction and Quality Assessment
Get Radiology Tree app to read full this article<
Meta-analysis
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Result
Get Radiology Tree app to read full this article<
Table 1
Characteristics of Studies Included in Meta-analysis
Study ID First Author (Ref.) Year Patients ( n ) Nation Lesion Number Study Design Blind b Value Field Strength QUADAS Score DWI Positivity Cutoff (ADC × 10 3 mm/s 2 ) 1 Tondo 2011 34 Italy 34 Retrospective ND 500\1000 1.5 10 ADC 1.25 2 Regier 2011 20 Germany 40 Retrospective ND ND 1.5 10 ADC ND 3 Ohba 2011 58 Japan 76 Prospective Y 1000 1.5 12 ADC 1.1 4 Liu 2010 62 China 66 ND Y 500 1.5 11 ADC 1.4 5 Koyama 2010 32 Japan 33 ND ND 1000 1.5 11 ADC 1.4 6 Uto 2009 28 Japan 28 Prospective ND 1000 1.5 10 LSR/ADC 0.834 7 Tanaka 2009 46 Japan 46 Retrospective Y ND 1.5 12 LSR ND 8 Ohba 2009 110 Japan 124 Retrospective Y 1000 1.5 11 ADC 1.4 9 Satoh 2008 51 Japan 54 ND Y 1000 1.5 13 LSR ND 10 Mori 2008 104 Japan 140 Prospective Y 1000 1.5 11 ADC 1.1 11 Wu 2010 61 China 61 ND Y 500 3 9 ADC 1.38 12 Wang 2008 56 China 56 ND Y 300\500\900 3 9 LMR ND 13 Chen 2011 58 China 58 ND Y 300\600 1.5 10 LSR/LMR ND 14 Cai 2011 97 China 97 Retrospective Y 600 1.5 9 ADC 1.5 15 Li 2011 116 China 120 ND Y 500\1000 3 9 ADC 1.47 16 Deng 2012 77 China 77 ND Y 500 1.5 9 ADC 1.49 17 Gümüştaş 2012 67 Turkey 67 Prospective Y 500\1000 1.5 11 LMR ND
ADC, apparent diffusion coefficient; DWI, diffusion-weighted magnetic resonance imaging; LMR, signal intensity ratio of lesion in muscle; LSR, signal intensity ratio of lesion in spinal cord; ND, no data; QUADAS, quality assessment of diagnostic studies; Y, yes.
Table 2
Raw Data of Diagnostic Performance of Studies Included in This Meta-analysis
Study ID First Author (Ref.) Year TP FP FN TN 1 Tondo 2011 27 0 3 4 2 Regier 2011 24 1 3 12 3 Ohba 2011 53 2 5 16 4 Liu 2010 45 3 9 9 5 Koyama 2010 13 2 10 8 6 Uto 2009 15 1 3 9 7 Tanaka 2009 32 3 1 10 8 Ohba 2009 70 1 26 27 9 Satoh 2008 32 7 4 11 10 Mori 2008 74 1 32 33 11 Wu 2010 33 4 5 19 12 Wang 2008 33 6 5 12 13 Chen 2011 31 11 7 9 14 Cai 2011 52 7 9 29 15 Li 2011 80 3 20 17 16 Deng 2012 48 7 3 19 17 Gümüştaş 2012 46 5 2 14
FN, false negative; FP, false positive; TN, true negative; TP, true positive.
Get Radiology Tree app to read full this article<
Threshold Effect Analysis
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Diagnostic Accuracy of DWI
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Meta-regression and Subgroup Analyses
Get Radiology Tree app to read full this article<
Table 3
Result of Meta-regression Analysis
Variable Coefficient Standard Error_P_ DOR 95% CI Study design 0.975 0.289 .006 2.65 1.41–4.97 Blind 0.237 0.633 .715 1.27 0.32–5.04 Field strength 0.347 0.345 .334 1.42 0.67–3.01 DWI positivity −0.656 0.416 .141 0.52 0.21–1.28
CI, confidence interval; DWI, diffusion-weighted magnetic resonance imaging; DOR, diagnostic odds ratio.
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Evaluation of Clinical Utility
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Publication Bias
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Discussion
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Conclusion
Get Radiology Tree app to read full this article<
Acknowledgments
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
References
1. Travis W.D., Brambilla E., Riely G.J.: New pathologic classification of lung cancer: relevance for clinical practice and clinical trials. J Clin Oncol 2013; 31: pp. 992-1001.
2. Boiselle P.M.: Computed tomography screening for lung cancer. JAMA 2013; 309: pp. 1163-1170.
3. Bomanji J., Almuhaideb A., Zumla A.: Combined PET and X-ray computed tomography imaging in pulmonary infections and inflammation. Curr Opin Pulm Med 2011; 17: pp. 197-205.
4. London K., Stege C., Cross S., et. al.: 18F-FDG PET/CT compared to conventional imaging modalities in pediatric primary bone tumors. Pediatr Radiol 2012; 42: pp. 418-430.
5. Ozgul M.A., Uysal M.A., Kadakal F., et. al.: [Comparison of computed tomography and magnetic resonance imaging to diagnose brain metastasis in non-small cell lung cancer]. Tuberk Toraks 2006; 54: pp. 229-234. [Article in Turkish]
6. Puderbach M., Kauczor H.U.: Can lung MR replace lung CT?. Pediatr Radiol 2008; 38: pp. S439-S451.
7. Razek A.A., Fathy A., Gawad T.A.: Correlation of apparent diffusion coefficient value with prognostic parameters of lung cancer. J Comput Assist Tomogr 2011; 35: pp. 248-252.
8. D’Ippolito G., Torres L.R., Saito Filho C.F., et. al.: CT and MRI in monitoring response: state-of-the-art and future developments. Q J Nucl Med Mol Imaging 2011; 55: pp. 603-619.
9. Koyama T., Tamai K., Togashi K.: Current status of body MR imaging: fast MR imaging and diffusion-weighted imaging. Int J Clin Oncol 2006; 11: pp. 278-285.
10. Wang W., Xu J.Z., Zhang T., et. al.: Diffusion-weighted magnetic resonance imaging with short T1 inversion recovery-echo planar imaging combined with dual-head coincidence single photon emission computed tomography for diagnosing solitary pulmonary nodule. Chin Med J (Engl) 2010; 123: pp. 3717-3721.
11. Whiting P., Rutjes A.W., Reitsma J.B., et. al.: The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Medical Res Methodol 2003; 3: pp. 25.
12. Zamora J., Abraira V., Muriel A., et. al.: Meta-DiSc: a software for meta-analysis of test accuracy data. BMC Medical Res Methodol 2006; 6: pp. 31.
13. Sinkus R., Van Beers B.E., Vilgrain V., et. al.: Apparent diffusion coefficient from magnetic resonance imaging as a biomarker in oncology drug development. Eur J Cancer 2012; 48: pp. 425-431.
14. Santos M.K., Elias J., Mauad F.M., et. al.: Magnetic resonance imaging of the chest: current and new applications, with an emphasis on pulmonology. J Bras Pneumol 2011; 37: pp. 242-258.
15. Liu H., Liu Y., Yu T., et. al.: Usefulness of diffusion-weighted MR imaging in the evaluation of pulmonary lesions. Eur Radiol 2010; 20: pp. 807-815.
16. Uto T., Takehara Y., Nakamura Y., et. al.: Higher sensitivity and specificity for diffusion-weighted imaging of malignant lung lesions without apparent diffusion coefficient quantification. Radiology 2009; 252: pp. 247-254.
17. Chen A.P., li H.M., Liu S.Y., et. al.: Differentiation of malignant and benign pulmonary lesions with diffusion-weighted imaging. Chin Comput Med Imaging 2010; 16: pp. 206-210.
18. Wang M.J., Zhang W., Zhang N., et. al.: Diagnostic value of 3.0 T magnetic resonance diffusion-weighted imaging in benign and malignant lung tumors. Acta Academiae Medicinae Jiangxi 2011; 51: pp. 14-19.
19. Wu L.M., Xu J.R., Hua J., et. al.: Can diffusion-weighted imaging be used as a reliable sequence in the detection of malignant pulmonary nodules and masses?. Magn Reson Imaging 2013; 31: pp. 235-246.
20. Chen L., Zhang J., Bao J., et. al.: Meta-analysis of diffusion-weighted MRI in the differential diagnosis of lung lesions. J Magn Reson Imaging 2013; 37: pp. 1351-1358.
21. Mori T., Nomori H., Ikeda K., et. al.: Diffusion-weighted magnetic resonance imaging for diagnosing malignant pulmonary nodules/masses: comparison with positron emission tomography. J Thorac Oncol 2008; 3: pp. 358-364.
22. Usuda K., Zhao X.T., Sagawa M., et. al.: Diffusion-weighted imaging is superior to positron emission tomography in the detection and nodal assessment of lung cancers. Ann Thorac Surg 2011; 91: pp. 1689-1695.
23. Satoh S., Kitazume Y., Ohdama S., et. al.: Can malignant and benign pulmonary nodules be differentiated with diffusion-weighted MRI?. AJR Am J Roentgenol 2008; 191: pp. 464-470.
24. Gümüştaş S., Inan N., Akansel G., et. al.: Differentiation of malignant and benign lung lesions with diffusion-weighted MR imaging. Radiol Oncol 2012; 46: pp. 106-113.
25. Cronin P., Dwamena B.A., Kelly A.M., et. al.: Solitary pulmonary nodules and masses: a meta-analysis of the diagnostic utility of alternative imaging tests. Eur Radiol 2008; 18: pp. 1840-1856.
26. Tondo F., Saponaro A., Stecco A., et. al.: Role of diffusion-weighted imaging in the differential diagnosis of benign and malignant lesions of the chest-mediastinum. Radiol Med 2011; 116: pp. 720-733.
27. Regier M., Schwarz D., Henes F.O., et. al.: Diffusion-weighted MR-imaging for the detection of pulmonary nodules at 1.5 Tesla: intraindividual comparison with multidetector computed tomography. J Med Imaging Radiat Oncol 2011; 55: pp. 266-274.
28. Ohba Y., Nomori H., Mori T., et. al.: Diffusion-weighted magnetic resonance for pulmonary nodules: 1.5 vs. 3 Tesla. Asian Cardiovasc Thorac Ann 2011; 19: pp. 108-114.
29. Koyama H., Ohno Y., Aoyama N., et. al.: Comparison of STIR turbo SE imaging and diffusion-weighted imaging of the lung: capability for detection and subtype classification of pulmonary adenocarcinomas. Eur Radiol 2010; 20: pp. 790-800.
30. Tanaka R., Horikoshi H., Nakazato Y., et. al.: Magnetic resonance imaging in peripheral lung adenocarcinoma: correlation with histopathologic features. J Thorac imaging 2009; 24: pp. 4-9.
31. Ohba Y., Nomori H., Mori T., et. al.: Is diffusion-weighted magnetic resonance imaging superior to positron emission tomography with fludeoxyglucose F 18 in imaging non-small cell lung cancer?. J Thorac Cardiovasc Surg 2009; 138: pp. 439-445.
32. Wu W.H., Chen J.J., Xu J.R., et. al.: The preliminary study of MR diffusion weighted imaging with background body signal suppression on pulmonary diseases. Zhonghua Fang She Xue Za Zhi 2008; 42: pp. 56-59.
33. Cai C.X., Zhao S.S., Lin L.P., et. al.: The value of MR diffusion weighted imaging with STIR-EPI sequence for differentiating benign from malignant pulmonary nodules. J Pract Med Imaging 2011; 12: pp. 358-361.
34. Li W.D., Li D., Liu H.D., et. al.: 3.0T MR diffusion-weighted imaging: evaluating diagnosis potency of pulmonary solid benign lesions and malignant tumors and optimizing b value. Zhongguo Fei Ai Za Zhi 2011; 14: pp. 853-857.
35. Deng Q.M., Qiu W.J., Zhang P.P., et. al.: The value on distinguishing pulmonary malignant tumors from benign lesions by diffusion-weighted imaging. Chinese CT and MRR magazine 2012; 10: pp. 35-37.