Rationale and Objectives
Acoustic droplet vaporization (ADV) shows promise for spatial control and acceleration of thermal lesion production. The investigators hypothesized that microbubbles generated by ADV could enhance high-intensity focused ultrasound (HIFU) thermal ablation by controlling and increasing local energy absorption.
Materials and Methods
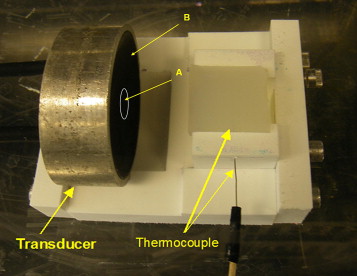
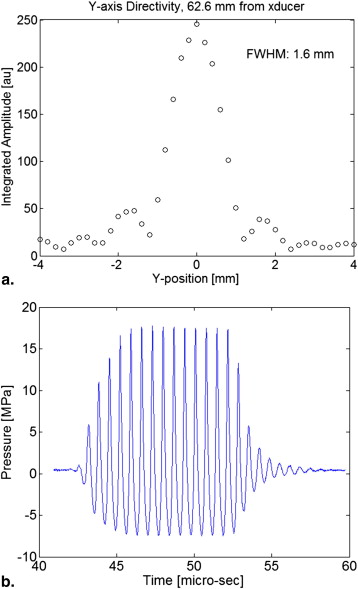
Thermal lesions were produced in tissue-mimicking phantoms using focused ultrasound (1.44 MHz) with a focal intensity of 4000 W · cm −2 in degassed water at 37°C. The average lesion volume was measured by visible change in optical opacity and by T2-weighted magnetic resonance imaging. In addition, in vivo HIFU lesions were generated in a canine liver before and after an intravenous injection of droplets with a similar acoustic setup.
Results
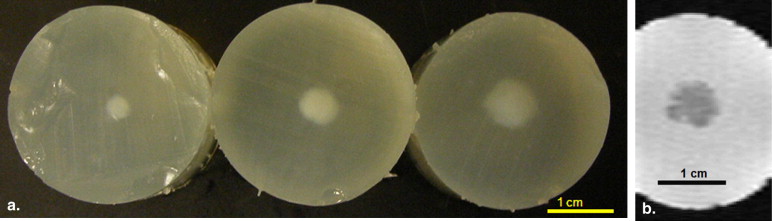
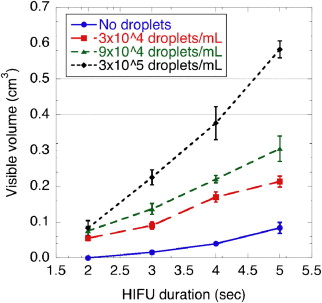
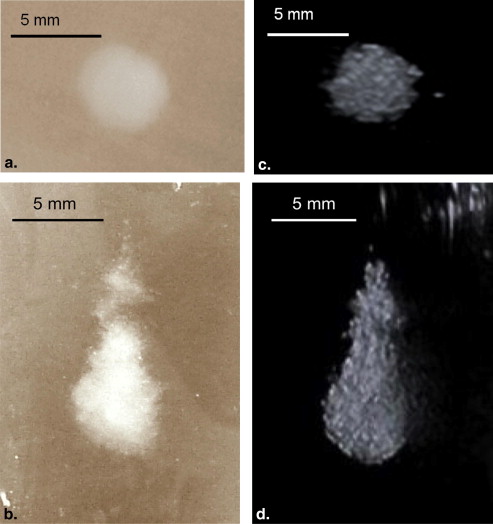
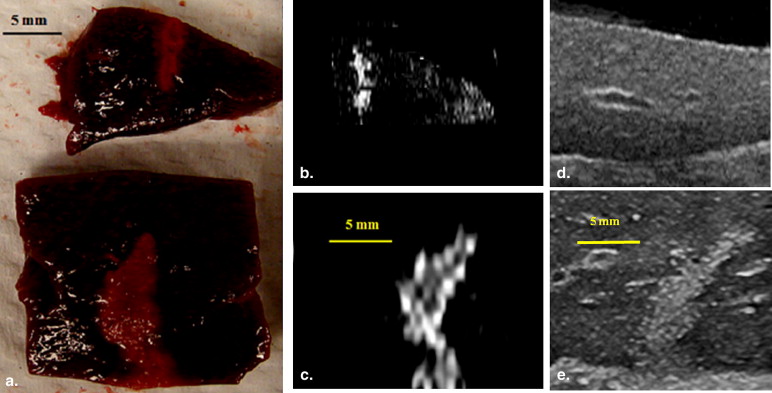
Thermal lesions were sevenfold larger in phantoms containing droplets (3 × 10 5 droplets/mL) compared to phantoms without droplets. The mean lesion volume with a 2-second HIFU exposure in droplet-containing phantoms was comparable to that made by a 5-second exposure in phantoms without droplets. In the in vivo study, the average lesion volumes without and with droplets were 0.017 ± 0.006 cm 3 (n = 4; 5-second exposure) and 0.265 ± 0.005 cm 3 (n = 3; 5-second exposure), respectively, a factor of 15 difference. The shape of ADV bubbles imaged with B-mode ultrasound was very similar to the actual lesion shape as measured optically and by magnetic resonance imaging.
Conclusion
ADV bubbles may facilitate clinical HIFU ablation by reducing treatment time or requisite in situ total acoustic power and provide ultrasonic imaging feedback of the thermal therapy.
Each year, >500,000 new cases of liver cancer are diagnosed worldwide, and the incidence is continuously increasing in the United States . Although surgical resection and liver transplantation are curative , only 20% to 30% of patients are suitable for surgery, because of contraindications and a limited number of donors . Overall, the 5-year survival rates of liver cancer are <10% . For these reasons, minimally invasive thermal therapies, including radiofrequency ablation and high-intensity focused ultrasound (HIFU) have been developed and are currently being used or evaluated for the treatment of liver masses. HIFU therapy is a noninvasive technique wherein focused ultrasound beams are emitted from a high-powered transducer to thermally ablate a tissue volume inside the body. HIFU systems commonly operate in a frequency range of 1 to 5 MHz, generating high focal intensities up to 30 kW · cm −2 . Such intensities can increase the tissue temperature beyond 60°C within the focal volume in seconds, resulting in irreversible protein denaturation, cell destruction, and associated coagulative necrosis . Two principal mechanisms, direct absorption of the transmitted pressure wave and acoustic cavitation, may cause tissue heating synergistically . The initial applications of HIFU on biologic tissues were proposed by Lynn et al . Later, Burov suggested using HIFU to treat malignant tumors. The bioeffects and specific properties of focused ultrasound on tissues have been investigated in further studies , and the physical principles associated with HIFU are becoming better understood .
HIFU treatment has distinct advantages over other focal ablative therapies. It is noninvasive and nonionizing. The focused energy is sufficient to induce thermal coagulation in a small volume within seconds, so the blood perfusion effects are minimized . However, a major difficulty has been creating homogenous, reproducible, and uniformly shaped lesions and producing these in such a manner as to treat large volumes at a rate that does not damage overlying tissues but achieves full treatment in a clinically manageable time period.
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Materials and methods
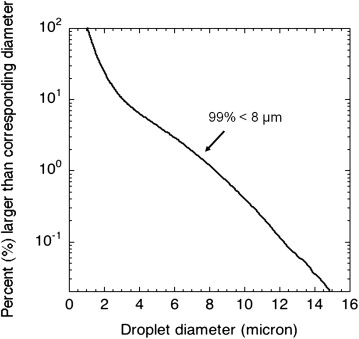
Perfluoropentane Droplet Preparation
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Tissue-mimicking Phantom Study
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
In Vivo HIFU in Canine Liver
Animal preparation
Get Radiology Tree app to read full this article<
HIFU lesion generation
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Lesion Size Estimation Using Ultrasound, Visible Macroscopic Imaging, and Magnetic Resonance Imaging (MRI)
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Statistical Analysis of Thermal Lesion Volume
Get Radiology Tree app to read full this article<
Results
In Vitro Phantom Study
Get Radiology Tree app to read full this article<
Table 1
Comparison of Lesion Volume Measured by MRI and Visible Macroscopic Imaging with 4 Seconds of HIFU Exposure
Droplet Concentration (droplets/mL) MRI Volume (cm 3 ) Optical Volume (cm 3 ) 0 (n = 8) 0.05 ± 0.01 0.04 ± 0.01 10 4 (n = 5) 0.10 ± 0.01 0.12 ± 0.02 10 5 (n = 8) 0.29 ± 0.05 0.30 ± 0.05 10 6 (n = 8) No lesion No lesion
At the concentration of 10 6 droplets/mL, copious acoustic droplet vaporization bubbles were observed, but no lesions were detected by either visible, macroscopic imaging or MRI, possibly because of the backscatter from the high-density acoustic droplet vaporization bubbles advancing the droplet vaporization proximally to shadow the focus.
HIFU, high-intensity focused ultrasound; MRI, magnetic resonance imaging.
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
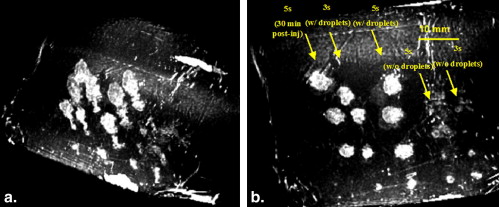
In Vivo Liver Study
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Discussion
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Conclusions
Get Radiology Tree app to read full this article<
Acknowledgments
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
References
1. Boyle P.Levin B.World cancer report 2008.2008.IARC PressLyon, France:
2. Yamasaki S., Hasegawa H., Makuuchi M., et. al.: Choice of treatments for small hepatocellular carcinoma: hepatectomy, embolization or ethanol injection. J Gastroenterol Hepatol 1991; 6: pp. 408-413.
3. Molinari M., Helton S.: Hepatic resection versus radiofrequency ablation for hepatocellular carcinoma in cirrhotic individuals not candidates for liver transplantation: a Markov model decision analysis. Am J Surg 2009; 198: pp. 396-406.
4. American Cancer Society: Cancer facts and figures.2004.American Cancer SocietyAtlanta, GA
5. Hill C.R., ter Haar G.R.: Review article: high intensity focused ultrasound—potential for cancer treatment. Br J Radiol 1995; 68: pp. 1296-1303.
6. Coussios C.C., Roy R.A.: Applications of acoustics and cavitation to noninvasive therapy and drug delivery. Annu Rev Fluid Mech 2008; 40: pp. 395-420.
7. Lynn J.G., Zwemer R.L., Chick A.J., et. al.: A new method for the generation and use of focused ultrasound in experimental biology. J Gen Physiol 1942; 26: pp. 179-193.
8. Burov A.K.: High-intensity ultrasonic vibrations for action on animal and human malignant tumours. Dokl Akad Nauk SSSR 1956; 106: pp. 239-241.
9. Bamber J.C., Hill C.R.: Ultrasonic attenuation and propagation speed in mammalian tissues as a function of temperature. Ultrasound Med Biol 1979; 5: pp. 149-157.
10. Parker K.J.: Ultrasonic attenuation and absorption in liver tissue. Ultrasound Med Biol 1983; 9: pp. 363-369.
11. Frizzell L.A.: Threshold dosages for damage to mammalian liver by high-intensity focused ultrasound. IEEE Trans Ultrason Ferroelectr Freq Control 1988; 35: pp. 578-581.
12. ter Haar G., Sinnett D., Rivens I.: High intensity focused ultrasound—a surgical technique for the treatment of discrete liver tumours. Phys Med Biol 1989; 34: pp. 1743-1750.
13. ter Haar G., Coussios C.: High intensity focused ultrasound: physical principles and devices. Int J Hyperthermia 2007; 23: pp. 89-104.
14. Billard B.E., Hynynen K., Roemer R.B.: Effects of physical parameters on high temperature ultrasound hyperthermia. Ultrasound Med Biol 1990; 16: pp. 409-420.
15. Zelkovic P.F., Resnick M.I.: Renal radiofrequency ablation: clinical status 2003. Curr Opin Urol 2003; 13: pp. 199-202.
16. Chavrier F., Chapelon J.Y., Gelet A., et. al.: Modeling of high-intensity focused ultrasound-induced lesions in the presence of cavitation bubbles. J Acoust Soc Am 2000; 108: pp. 432-440.
17. Rabkin B.A., Zderic V., Vaezy S.: Hyperecho in ultrasound images of HIFU therapy: involvement of cavitation. Ultrasound Med Biol 2005; 31: pp. 947-956.
18. Coussios C.C., Farny C.H., Haar G.T., et. al.: Role of acoustic cavitation in the delivery and monitoring of cancer treatment by high-intensity focused ultrasound (HIFU). Int J Hyperthermia 2007; 23: pp. 105-120.
19. Farny C.H., Glynn Holt R., Roy R.A.: The correlation between bubble-enhanced HIFU heating and cavitation power. IEEE Trans Biomed Eng 2010; 57: pp. 175-184.
20. Canney M.S., Khokhlova V.A., Bessonova O.V., et. al.: Shock-induced heating and millisecond boiling in gels and tissue due to high intensity focused ultrasound. Ultrasound Med Biol 2010; 36: pp. 250-267.
21. Yang X., Roy R.A., Holt R.G.: Bubble dynamics and size distributions during focused ultrasound insonation. J Acoust Soc Am 2004; 116: pp. 3423-3431.
22. Stride E.P., Coussios C.C.: Cavitation and contrast: the use of bubbles in ultrasound imaging and therapy. Proc Inst Mech Eng H 2010; 224: pp. 171-191.
23. Zhang S., Wan M., Zhong H., et. al.: Dynamic changes of integrated backscatter, attenuation coefficient and bubble activities during high-intensity focused ultrasound (HIFU) treatment. Ultrasound Med Biol 2009; 35: pp. 1828-1844.
24. Umemura S., Kawabata K., Sasaki K.: In vivo acceleration of ultrasonic tissue heating by microbubble agent. IEEE Trans Ultrason Ferroelectr Freq Control 2005; 52: pp. 1690-1698.
25. Tung Y.S., Liu H.L., Wu C.C., et. al.: Contrast-agent-enhanced ultrasound thermal ablation. Ultrasound Med Biol 2006; 32: pp. 1103-1110.
26. Kripfgans O.D., Fowlkes J.B., Miller D.L., et. al.: Acoustic droplet vaporization for therapeutic and diagnostic applications. Ultrasound Med Biol 2000; 26: pp. 1177-1189.
27. Rapoport N., Gao Z.G., Kennedy A.: Multifunctional nanoparticles for combining ultrasonic tumor imaging and targeted chemotherapy. J Natl Cancer Inst 2007; 99: pp. 1095-1106.
28. Kawabata K., Sugita N., Yoshikawa H., et. al.: Nanoparticles with multiple perfluorocarbons for controllable ultrasonically induced phase shifting. Jpn J Appl Phys Part 1 Reg Papers Brief Commun Rev Papers 2005; 44: pp. 4548-4552.
29. Kripfgans O.D., Orifici C.M., Carson P.L., et. al.: Acoustic droplet vaporization for temporal and spatial control of tissue occlusion: a kidney study. IEEE Trans Ultras Ferroel Freq Control 2005; 52: pp. 1101-1110.
30. Lo A.H., Kripfgans O.D., Carson P.L., et. al.: Spatial control of gas bubbles and their effects on acoustic fields. Ultrasound Med Biol 2006; 32: pp. 95-106.
31. Zhang M., Fabiilli M.L., Haworth K.J., et. al.: Initial investigation of acoustic droplet vaporization for occlusion in canine kidney. Ultrasound Med Biol 2010; 36: pp. 1691-1703.
32. Kripfgans O.D.: Acoustic droplet vaporization for diagnostic and therapeutic applications.2002.University of MichiganAnn Arbor
33. Zhang P., Porter T.: An in vitro study of a phase-shift nanoemulsion: a potential nucleation agent for bubble-enhanced HIFU tumor ablation. Ultrasound Med Biol 2010; 36: pp. 1856-1866.
34. Fabiilli M.L., Haworth K.J., Fakhri N.H., et. al.: The role of inertial cavitation in acoustic droplet vaporization. IEEE Trans Ultrason Ferroelectr Freq Control 2009; 56: pp. 1006-1017.
35. Takegami K., Kaneko Y., Watanabe T., et. al.: Polyacrylamide gel containing egg white as new model for irradiation experiments using focused ultrasound. Ultrasound Med Biol 2004; 30: pp. 1419-1422.
36. Flaim S.F.: Pharmacokinetics and side effects of perfluorocarbon-based blood substitutes. Artif Cells Blood Substit Immobilization Biotechnol 1994; 22: pp. 1043-1054.
37. Lambelet P., Ducret F., Leuba J.L., et. al.: Low-field nuclear magnetic resonance relaxation study of the thermal denaturation of transferrins. J Agric Food Chem 1991; 39: pp. 287-292.
38. Weld K.J., Landman J.: Comparison of cryoablation, radiofrequency ablation and high-intensity focused ultrasound for treating small renal tumours. BJU Int 2005; 96: pp. 1224-1229.
39. Holt R.G., Roy R.A.: Measurements of bubble-enhanced heating from focused, MHz-frequency ultrasound in a tissue-mimicking material. Ultrasound Med Biol 2001; 27: pp. 1399-1412.
40. Lafon C., Murillo-Rincon A., Goldenstedt C., et. al.: Feasibility of using ultrasound contrast agents to increase the size of thermal lesions induced by non-focused transducers: in vitro demonstration in tissue mimicking phantom. Ultrasonics 2009; 49: pp. 172-178.
41. Lele P.P.: Effects of ultrasound on “solid” mammalian tissues and tumors in vivo.Repacholi M.H.Grondolfo M.Rindi A.Ultrasound: medical applications, biological effects and hazard potential.1987.PlenumNew York:pp. 273-306.
42. Hynynen K.: The threshold for thermally significant cavitation in dog’s thigh muscle in vivo. Ultrasound Med Biol 1991; 17: pp. 157-169.
43. Clarke R.L., ter Haar G.R.: Temperature rise recorded during lesion formation by high-intensity focused ultrasound. Ultrasound Med Biol 1997; 23: pp. 299-306.
44. National Council on Radiation Protection and Measurements: Exposure criteria for medical ultrasound. Part 1: exposure based on thermal mechanisms.1992.National Council on Radiation Protection and MeasurementsBethesda, MD
45. Wootton J.H., Ross A.B., Diederich C.J.: Prostate thermal therapy with high intensity transurethral ultrasound: the impact of pelvic bone heating on treatment delivery. Int J Hyperthermia 2007; 23: pp. 609-622.
46. National Council on Radiation Protection and Measurements: Exposure criteria for medical diagnostic ultrasound: II. Criteria based on all known mechanisms. NCRP Report No 140.2002.National Council on Radiation Protection and MeasurementsBethesda, MD
47. Clark L.C., Hoffmann R.E., Davis S.L.: Response of the rabbit lung as a criterion of safety for fluorocarbon breathing and blood substitutes. Biomater Artif Cells Immobilization Biotechnol 1992; 20: pp. 1085-1099.
48. Schutt E., Barber P., Fields T., et. al.: Proposed mechanism of pulmonary gas trapping (PGT) following intravenous perfluorocarbon emulsion administration. Artif Cells Blood Substit Immobilization Biotechnol 1994; 22: pp. 1205-1214.
49. Gettman M.T., Lotan Y., Corwin T.S., et. al.: Radiofrequency coagulation of renal parenchyma: Comparison of effects of energy generators on treatment efficacy. J Endourol 2002; 16: pp. 83-88.
50. Klingler H.C., Susani M., Seip R., et. al.: A novel approach to energy ablative therapy of small renal tumors: laparoscopic high-intensity focused ultrasound. Eur Urol 2008; 53: pp. 810-818.
51. Bradley W.G.: MR-guided focused ultrasound: a potentially disruptive technology. J Am Coll Radiol 2009; 6: pp. 510-513.
52. Paskalev K., Feigenberg S., Jacob R., et. al.: Target localization for post-prostatectomy patients using CT and ultrasound image guidance. J Appl Clin Medical Phys 2005; 6: pp. 40-49.
53. Jaffray D., Kupelian P., Djemil T., et. al.: Review of image-guided radiation therapy. Expert Rev Anticancer Ther 2007; 7: pp. 89-103.
54. Kripfgans O.D., Fabiilli M.L., Carson P.L., et. al.: On the acoustic vaporization of micrometer-sized droplets. J Acoust Soc Am 2004; 116: pp. 272-281.
55. Kohler M.O., Mougenot C., Quesson B., et. al.: Volumetric HIFU ablation under 3D guidance of rapid MRI thermometry. Med Phys 2009; 36: pp. 3521-3535.
56. Terraz S., Cernicanu A., Lepetit-Coiffe M., et. al.: Radiofrequency ablation of small liver malignancies under magnetic resonance guidance: progress in targeting and preliminary observations with temperature monitoring. Eur Radiol 2010; 20: pp. 886-897.