Rationale and objectives
To determine the diagnostic value of apparent diffusion coefficient (ADC) maps in the assessment of response to chemotherapy in patients with multiple myeloma (MM).
Materials and methods
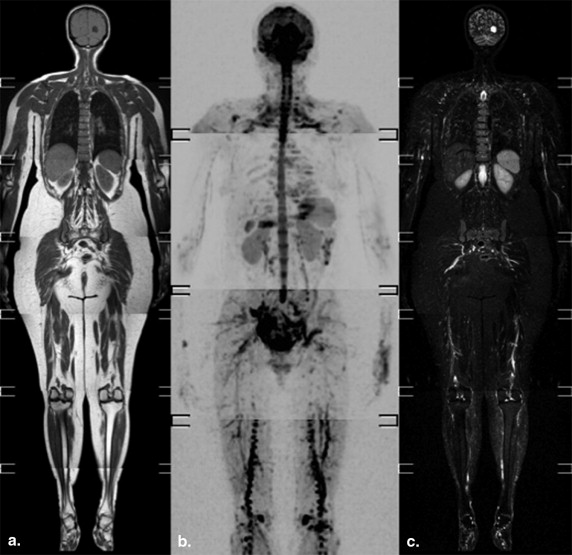
Fourteen patients (seven women) with MM underwent whole-body magnetic resonance imaging (WB-MRI) study on a 1.5T scanner, before and after chemotherapy. DWI with background body signal suppression (DWIBS) sequences ( b values: 0, 500, and 1000 mm 2 /sec) were qualitatively analyzed, along with T1 turbo spine echo and short tau inversion recovery T2-weighted images, to evaluate bone lesions. On ADC maps, regions of interest were manually drawn along contours of lesions. The ADC values percentage variation (ΔADC) before (MR1) and after (MR2) chemotherapy were calculated and compared between responders (11 of 14) and nonresponders (3 of 14). The percentage of plasma cells by the means of the bone marrow aspirate was evaluated as parameter for response to chemotherapy.
Results
Twenty-four lesions, hyperintense on DWIBS as compared to normal bone marrow, were evaluated. In responder group, the mean ADC values were 0.63 ± 0.24 × 10 -3 mm 2 /s on MR1 and 1.04 ± 0.46 × 10 -3 mm 2 /s on MR2; partial or complete signal intensity decrease during follow-up on DWIBS was found along with a reduction of plasma cells infiltration in the bone marrow. The mean ADC values for nonresponders were 0.61 ± 0.05 × 10 -3 mm 2 /s on MR1 and 0.69 ± 0.09 × 10 -3 mm 2 /s on MR2. The mean variation of ΔADC in responders (Δ = 66%) was significantly different ( P < .05) than in nonresponders (Δ = 15%).
Conclusions
WB-MRI with DWIBS sequences, by evaluating posttreatment changes of ADC values, might represent a complementary diagnostic tool in the assessment of response to chemotherapy in MM patients.
In the past decades, magnetic resonance imaging (MRI) has shown a high diagnostic and prognostic value in monoclonal plasma cell disorders . It has also been included as additional imaging tool for diagnosis and staging protocols of multiple myeloma (MM) , but, to date, there is insufficient evidence to recommend routine MRI after treatment because most of the findings are not specific .
In MM follow-up, the role of imaging is still limited, relying on skeletal survey although bone marrow aspirate and laboratory parameters (serum and urinary M-protein measurements) are the mainstay of the evaluation of response to chemotherapy. According to the most recent guidelines, plain films should be repeated after clinical or laboratory evidence of disease progression , but on conventional radiographs, lytic bone lesions rarely demonstrate signs of healing. Moreover, the evidence of new vertebral compressions may not indicate disease progression, occurring even after an effective therapy . Therefore, an accurate imaging technique able to detect treatment-related changes could increase the ability to monitor MM patients, even those with reduced or absent M-protein secretion.
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Materials and methods
Study Population
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
MRI Technique
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Table 1
Whole-Body Magnetic Resonance Imaging Protocol (1.5T), Acquired with Patient Positioned Supine and Using Built-In Body Coil Combined with Stepping Table Technique
Sequence T2 STIR T1 TSE DWIBS Slice thickness, mm 4 4 4 Plane of acquisition Coronal (C) and sagittal (S) Coronal (C) and sagittal (S) Axial Range of scan_Coronal_ : skull vertex to feet. Sagittal : axial skeleton_Coronal_ : skull vertex to feet. Sagittal : axial skeleton Skull vertex to feet_b_ values / / 0, 500, 1000 s/mm 2 TR 1400 1000 1400 TE 64 17.50 66 TI 165 — 180 FA 90° 90° 90° NSA 2 1 2 Voxel size C: 1.27 × 1.82 mm 3 S: 1.17 × 1.53 mm 3 C: 1.58 × 1.97 mm 3 S: 1.38 × 1.75 mm 3 5 × 4.97 mm 3
DWIBS, diffusion-weighted imaging with background body signal suppression; FA, flip angle; NSA, number of signals acquired; STIR, short tau inversion recovery; TE, echo time; TI, inversion time; TR, repetition time; TSE, turbo spine echo.
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Patients and Imaging Analysis
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Hystopathologic Analysis
Get Radiology Tree app to read full this article<
Statistical Analysis
Get Radiology Tree app to read full this article<
Results
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Table 2
Apparent Diffusion Coefficient (ADC) Values and Percentage Variation of ADC (ΔADC%) of Evaluated Lesions (Both as Focal or Diffuse Involvement), in Responder Versus Nonresponder Patients, Before (ADC1) and After (ADC2) Chemotherapy
Lesions Responders Mean ± SD Nonresponders Mean ± SD Rank-Sum Test ( P Value <.05) ADC1/pretherapy (× 10 −3 mm 2 /s) 0.63 ± 0.24 0.61 ± 0.05 .71 ADC2/posttherapy (× 10 −3 mm 2 /s) 1.04 ± 0.46 0.69 ± 0.09 .18 ΔADC% 66% 15% .05
SD, standard deviation.
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Discussion
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
References
1. Terpos E., Moulopoulos L.A., Dimopoulos M.A.: Advances in imaging and the management of myeloma bone disease. J Clin Oncol 2011; 29: pp. 1907-1915.
2. Dimopoulos M., Terpos E., Comenzo R.L., et. al.: International myeloma working group (IMWG) consensus statement and guidelines regarding the current role of imaging techniques in the diagnosis and monitoring of multiple myeloma. Leukemia 2009; 23: pp. 1545-1556.
3. Sommer G., Klarhöfer M., Lenz C., et. al.: Signal characteristics of focal bone marrow lesions in patients with multiple myeloma using whole body T1w-TSE, T2w-STIR and diffusion-weighted imaging with background suppression. Eur Radiol 2011; 21: pp. 857-862.
4. Durie B.G.: The role of anatomic and functional staging in myeloma: description of Durie/Salmon plus staging system. Eur J Cancer 2006; 42: pp. 1539-1543.
5. D’Sa S., Abildgaard N., Tighe J., et. al.: Guidelines for the use of imaging in the management of myeloma. Br J Haematol 2007; 137: pp. 49-63.
6. Bird J.M., Owen R.G., D’Sa S., et. al.: Haemato-oncology Task Force of British Committee for Standards in Haematology (BCSH) and UK Myeloma Forum. Guidelines for the diagnosis and management of multiple myeloma 2011. Br J Haematol 2011; 154: pp. 32-75.
7. Horger M., Weisel K., Horger W., et. al.: Whole-body diffusion-weighted MRI with apparent diffusion coefficient mapping for early response monitoring in multiple myeloma: preliminary results. AJR Am J Roentgenol 2011; 196: pp. W790-W795.
8. Kwee T.C., Takahara T., Ochiai R., et. al.: Diffusion-weighted whole-body imaging with background body signal suppression (DWIBS): features and potential applications in oncology. Eur Radiol 2008; 18: pp. 1937-1952.
9. Kwee T.C., Takahara T., Ochiai R., et. al.: Whole-body diffusion-weighted magnetic resonance imaging. Eur J Radiol 2009; 70: pp. 409-417.
10. Bammer R.: Basic principles of diffusion-weighted imaging. Eur J Radiol 2003; 45: pp. 169-184.
11. Padhani A.R., Liu G., Koh D.M., et. al.: Diffusion-weighted magnetic resonance imaging as a cancer biomarker: consensus and recommendations. Neoplasia 2009; 11: pp. 102-125.
12. Takahara T., Imai Y., Yamashita T., et. al.: Diffusion weighted whole body imaging with background body signal suppression (DWIBS): technical improvement using free breathing, STIR and high resolution 3D display. Radiat Med 2004; 22: pp. 275-282.
13. Schaefer P.W., Grant P.E., Gonzalez R.G.: Diffusion-weighted MR imaging of the brain. Radiology 2000; 217: pp. 331-345.
14. Hosonuma T., Tozaki M., Ichiba N., et. al.: Clinical usefulness of diffusion-weighted imaging using low and high b-values to detect rectal cancer. Magn Reson Med Sci 2006; 5: pp. 173-177.
15. Yağci A.B., Ozari N., Aybek Z., et. al.: The value of diffusion-weighted MRI for prostate cancer detection and localization. Diagn Interv Radiol 2011; 17: pp. 130-134.
16. Fattahi R., Balci N.C., Perman W.H., et. al.: Pancreatic diffusion-weighted imaging (DWI): comparison between mass-forming focal pancreatitis (FP), pancreatic cancer (PC), and normal pancreas. J Magn Reson Imaging 2009; 29: pp. 350-356.
17. Fenchel M., Konaktchieva M., Weisel K., et. al.: Response assessment in patients with multiple myeloma during antiangiogenic therapy using arterial spin labeling and diffusion-weighted imaging: a feasibility study. Acad Radiol 2010; 17: pp. 1326-1333.
18. Messiou C., Giles S., Collins D.J., et. al.: Assessing response of myeloma bone disease with diffusion-weighted MRI. Br J Radiol 2012; 85: pp. 1198-1203.
19. Delorme S., Baur-Melnyk A.: Imaging in multiple myeloma. Eur J Radiol 2009; 70: pp. 401-408.
20. Baur A., Dietrich O., Reiser M.: Diffusion-weighted imaging of bone marrow: current status. Eur Radiol 2003; 13: pp. 1699-1708.
21. Thoeny H.C., Ross B.D.: Predicting and monitoring cancer treatment response with diffusion-weighted MRI. J Magn Reson Imaging 2010; 32: pp. 2-16.
22. Sun Y.S., Cui Y., Tang L., et. al.: Early evaluation of cancer response by a new functional biomarker: apparent diffusion coefficient. AJR Am J Roentgenol 2011; 197: pp. 23-29.
23. Cui Y., Zhang X.P., Sun Y.S., et. al.: Apparent diffusion coefficient: potential imaging biomarker for prediction and early detection of response to chemotherapy in hepatic metastases. Radiology 2008; 248: pp. 894-900.
24. Harry V.N., Semple S.I., Gilbert F.J., et. al.: Diffusion-weighted magnetic resonance imaging in the early detection of response to chemoradiation in cervical cancer. Gynecol Oncol 2008; 111: pp. 213-220.
25. Lee K.C., Bradley D.A., Hussain M., et. al.: A feasibility study evaluating the functional diffusion map as a predictive imaging biomarker for detection of treatment response in a patient with metastatic prostate cancer to the bone. Neoplasia 2007; 9: pp. 1003-1011.
26. Hayashida Y., Yakushiji T., Awai K., et. al.: Monitoring therapeutic responses of primary bone tumors by diffusion-weighted image: Initial results. Eur Radiol 2006; 16: pp. 2637-2643.
27. Messiou C., Collins D.J., Giles S., et. al.: Assessing response in bone metastases in prostate cancer with diffusion weighted MRI. Eur Radiol 2011; 21: pp. 2169-2177.
28. Byun W.M., Shin S.O., Chang Y., et. al.: Diffusion-weighted MR imaging of metastatic disease of the spine: assessment of response to therapy. Am J Neuroradiol 2002; 23: pp. 906-912.
29. Kim S.H., Lee J.Y., Lee J.M., et. al.: Apparent diffusion coefficient for evaluating tumor response to neoadjuvant chemoradiation therapy for locally advanced rectal cancer. Eur Radiol 2011; 21: pp. 987-995.
30. Ippolito D., Monguzzi L., Guerra L., et. al.: Response to neoadjuvant therapy in locally advanced rectal cancer: assessment with diffusion-weighted MR imaging and 18FDG PET/CT. Abdom Imaging 2012; 37: pp. 1032-1040.
![Figure 2, Whole-body magnetic resonance imaging (WB-MRI) study of a 67 years old female with histologically proven MM. (a–d) Coronal T1 turbo spine echo (TSE) (a) and short tau inversion recovery (STIR) T2 (b) weighted and axial diffusion-weighted imaging with background body signal suppression (DWIBS) ( c , b value = 1000 s/mm 2 ) images of the femurs. At time of diagnosis, the WB examination demonstrates a focal lesion in the right femoral diaphysis, characterized by hypointensity on T1 [ large arrow on (a) ] and high signal intensity both on STIR and DWIBS [ large and thin arrow on (b) and (c) , respectively]. The corresponding apparent diffusion coefficient (ADC) value on ADC map [ thin arrow on (d) ] was 0.68 × 10 −3 mm 2s. (e–h) Posttreatment coronal T1 TSE (e) and STIR T2 (f) weighted and axial DWBIS ( g , b value = 1000 s/mm 2 ) images. The primary lesion is still detectable on standard anatomical sequences, even if with a partial decrease of signal intensity on the fat sat image [ large arrows on (e) and (f) , respectively]. DWIBS shows a marked decrease of signal intensity alteration. The ADC value calculated on the corresponding ADC map [ thin arrow on (h) ] increased (0.85 × 10 −3 mm 2s), as neoplastic cellular load is reduced by therapy.](https://storage.googleapis.com/dl.dentistrykey.com/clinical/ApparentDiffusionCoefficientMapsIntegratedinWholeBodyMRIExaminationfortheEvaluationofTumorResponsetoChemotherapyinPatientswithMultipleMyeloma/1_1s20S1076633215002494.jpg)
![Figure 3, Pre- and post-chemotherapy evaluation in a 49-year-old woman with MM. (a–d) Sagittal T1 turbo spine echo (TSE) (a) and short tau inversion recovery (STIR) T2 (b) weighted and axial diffusion-weighted imaging with background body signal suppression (DWIBS) ( c , b value = 1000 s/mm 2 ) images show a diffuse pattern of infiltration of the dorsal axial skeleton, hypointense on T1 and slightly hyperintense on STIR sequences, and with marked high signal intensity on DWIBS image [ thin arrow on (c) ]. The apparent diffusion coefficient (ADC) value, calculated on the corresponding map at the level of D11 [ thin arrow on (d) ], was 0.57 × 10 −3 mm 2s. On the highest b value DWIBS image, also the spleen is still characterized by a high signal intensity [ curved arrow on (d) ]. (e–h) Sagittal T1 TSE (e) and STIR T2 (f) weighted and axial DWBIS ( g , b value = 1000 s/mm 2 ) images after chemotherapy demonstrate no significant change of signal intensity's alterations of bone marrow. Also, the corresponding ADC value measured on the ADC map (h) does not show any variation (0.61 × 10 −3 mm 2s), as per no response to the treatment.](https://storage.googleapis.com/dl.dentistrykey.com/clinical/ApparentDiffusionCoefficientMapsIntegratedinWholeBodyMRIExaminationfortheEvaluationofTumorResponsetoChemotherapyinPatientswithMultipleMyeloma/2_1s20S1076633215002494.jpg)