Rationale and Objectives
This study was designed to demonstrate the feasibility of the use of phase-contrast computed tomographic (CT) imaging for the identification of articular cartilage abnormalities of the knees in a mouse model of collagen-induced arthritis.
Materials and Methods
Arthritis was induced in nine male DBA/1 J mice by the intradermal injection of collagen. After 50 days, the nine mice were sacrificed, along with four mice that did not receive intradermal injections of collagen. Phase-contrast CT imaging using a microfocus x-ray source of the entire knee was performed. The images were evaluated by two blinded readers, and histopathologic grades were considered the reference standard. The phase-contrast CT images of cartilage were graded 0, I, or II. Evaluation of the grading agreement between the phase-contrast CT images and histopathologic findings was performed using correlation analysis.
Results
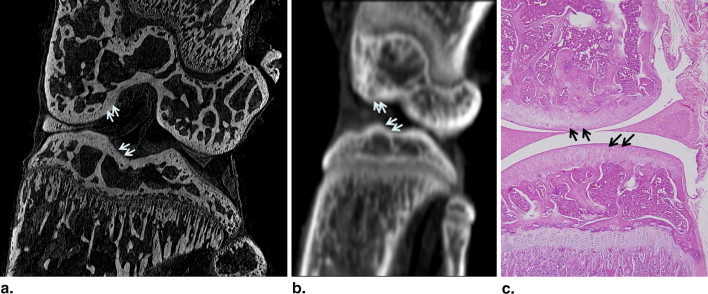
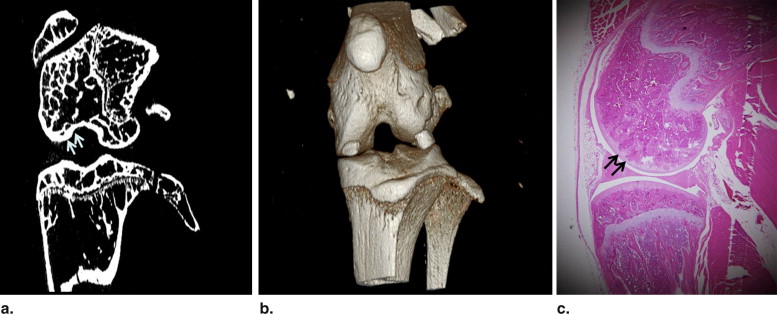
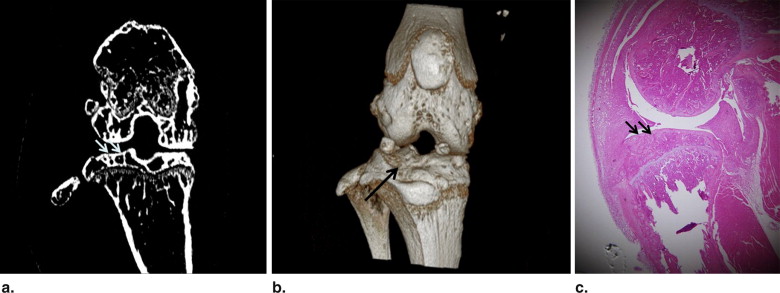
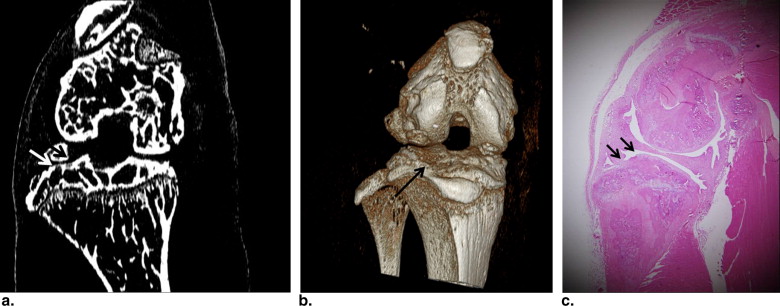
Phase-contrast CT images highly reflected the subchondral bone status in the assessment of articular cartilage abnormalities in the mouse model of collagen-induced arthritis. Three-dimensional reformed images showed the articular surface and subchondral bony status of the knee joints. On the basis of the histopathology of the 26 knee joints, 12 joints were grade 0, six joints were grade I, and eight joints were grade II. Grading agreement between the use of the phase-contrast CT images and histopathologic results was high ( r = 0.76).
Conclusions
Phase-contrast CT imaging using a microfocus x-ray source offers a promising tool for the assessment of articular cartilage abnormalities of the knees in a mouse model.
The early diagnosis of arthritis allows patients to begin treatment before disease progression leads to disability. For inflammatory arthritis, the articular cartilage is damaged before the subchondral bone . Many imaging methods have been used to evaluate inflammatory arthritis. Conventional radiography and computed tomographic (CT) imaging are very limited in the depiction and evaluation of articular cartilage directly. Until recently, magnetic resonance imaging has been the most promising tool for cartilage imaging, especially to obtain high-resolution images. However, alternative imaging techniques to evaluate cartilage should provide diagnostic benefits for clinical use and basic research. Thus, a new cartilage imaging method with excellent diagnostic performance and practical utility would be desirable.
Recently, phase-contrast x-ray imaging has been regarded as a new imaging method that provides soft-tissue discrimination at micrometer resolution, with lower radiation doses than required by conventional absorption techniques . Conventional x-ray imaging uses differences in the x-ray absorption coefficients of tissues, but the difference is small for soft tissue, resulting in poor image contrast. Phase-contrast x-ray imaging uses differences in the refractive indexes of different tissues, where the difference in the refractive index is high at the boundaries for different tissues, resulting in an edge enhancement effect . The phase shift cross-section is almost 1,000 times greater than the absorption cross-section, resulting in high image contrast . Several studies have demonstrated high image contrast of phase-contrast x-ray imaging using synchrotron radiation for cartilage and bone ( ), blood vessels , the breast ( ), and the lung . However, these studies remain almost completely restricted to the laboratory, and in particular, the studies have required the use of synchrotron radiation sources. Although a synchrotron radiation source can produce high-resolution images with short exposure times, such sources are available only at restricted facilities and are very inconvenient for users.
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Materials and methods
Murine Model of Collagen-induced Arthritis
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Phase-contrast CT System
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Image Analysis
Get Radiology Tree app to read full this article<
Histopathologic Correlation
Get Radiology Tree app to read full this article<
Statistical Analysis
Get Radiology Tree app to read full this article<
Results
Phantom Study of Phase-contrast CT Imaging
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Phase-contrast CT Imaging of Articular Cartilage of the Arthritis Model
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Phase-contrast CT Images Correlated with Histopathology
Get Radiology Tree app to read full this article<
Table 1
Comparison of the Histologic and Phase-contrast CT Imaging Grades of 26 Knee Joints
CT Grade Histologic Grade 0 I II Total 0 12 0 0 12 I 0 4 2 6 II 0 0 8 8 Total 12 4 10 26
CT, computed tomographic.
The correlation coefficient of the two independent measurements was r = 0.76.
Get Radiology Tree app to read full this article<
Discussion
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
References
1. Poole A.R.: An introduction to the pathophysiology of osteoarthritis. Front Biosci 1999; 4: pp. D662-D670.
2. Gold G.E., McCauley T.R., Gray M.L., et. al.: What’s new in cartilage?. Radiographics 2003; 23: pp. 1227-1242.
3. Lewis R.A.: Medical phase contrast x-ray imaging: current status and future prospects. Phys Med Biol 2004; 49: pp. 3573-3583.
4. Lewis R.A., Hall C.J., Hufton A.P., et. al.: X-ray refraction effects: application to the imaging of biological tissues. Br J Radiol 2003; 76: pp. 301-308.
5. Momose A., Takeda T., Itai Y.: Blood vessels: depiction at phase-contrast x-ray imaging without contrast agents in the mouse and rat-feasibility study. Radiology 2000; 217: pp. 593-596.
6. Mori K., Hyodo K., Shikano N., et. al.: First observation of small fractures on a human dried proximal phalanx by synchrotron x-ray interference radiography. Jpn J Appl Phys 1999; 38: pp. 1339-1341.
7. Mori K., Sekine N., Sato H., et. al.: Application of synchrotron x-ray imaging to phase objects in orthopedics. J Synchrotron Radiat 2002; 9: pp. 143-147.
8. Mollenhauer J., Aurich M.E., Zhong Z.: Diffraction-enhanced x-ray imaging of articular cartilage. Osteoarthritis Cartilage 2002; 10: pp. 163-171.
9. Li J., Zhong Z., Lidtke R.: Radiography of soft tissue of the foot and ankle with diffraction enhanced imaging. J Anat 2003; 202: pp. 463-470.
10. Ingal V.N., Beliaevskaya E.A., Brianskaya A.P., et. al.: Phase mammography—a new technique for breast investigation. Phys Med Biol 1998; 43: pp. 2555-2567.
11. Pisano E.D., Johnston R.E., Chapman D., et. al.: Human breast cancer specimens: diffraction-enhanced imaging with histologic correlation—improved conspicuity of lesion detail compared with digital radiography. Radiology 2000; 214: pp. 895-901.
12. Arfelli F., Bonvicini V., Bravin A., et. al.: Mammography with synchrotron radiation: phase-detection techniques. Radiology 2000; 215: pp. 286-293.
13. Suzuki Y., Yagi N., Uesugi K.: X-ray refraction-enhanced imaging and a method for phase retrieval for a simple object. J Synchrotron Radiat 2002; 9: pp. 160-165.
14. Shibata T., Nagano T.: Applying very high resolution microfocus x-ray CT and 3-D reconstruction to the human auditory apparatus. Nat Med 1996; 2: pp. 933-935.
15. Kashyap Y.S., Roy T., Sarkar P.S., et. al.: Characterization of pyrocarbon coated materials using laboratory based x-ray phase contrast imaging technique. Rev Sci Instrum 2007; pp. 78. 083703
16. Snigirev A., Snigireva I., Kohn V., et. al.: On the possibilities of x-ray phase contrast microimaging by coherent high-energy synchrotron radiation. Rev Sci Instrum 1995; 66: pp. 5486-5492.
17. Cloetens P., Barrett R., Baruchel J., et. al.: Phase objects in synchrotron radiation hard x-ray imaging. J Phys D Appl Phys 1996; 29: pp. 133-146.
18. Nugent K.A., Gureyev T.E., Cookson D.F., et. al.: Quantitative phase imaging using hard x-rays. Phys Rev Lett 1996; 77: pp. 2961-2964.
19. Foerster E., Goetz K., Zaumseil P.: Double crystal diffractometry for the characterization of targets for laser fusion experiments. Krist Tech 1980; 15: pp. 937-945.
20. Somenkov V.A., Tkalich A.K., Shil’stein S.: Refraction contrast in x-ray microscopy. Sov Phys Tech Phys 1991; 3: pp. 1309-1311.
21. Davis T.J., Gao D., Gureyev T.E., et. al.: Phase contrast imaging of weakly absorbing materials using hard x-rays. Nature 1995; 373: pp. 595-598.
22. Chapman D., ThomLinson W., Johnston R.E., et. al.: Diffraction enhanced x-ray imaging. Phys Med Biol 1997; 42: pp. 2015-2025.
23. Bravin A.: Exploiting the x-ray refraction contrast with an analyser: the state of the art. J Phys D 2003; 36: pp. A24-A29.
24. Parham C., Zhong Z., Connor D.M., et. al.: Design and implementation of a compact low-dose diffraction enhanced medical imaging system. Acad Radiol 2009; 16: pp. 911-917.
25. Muehleman C., Li J., Connor D., et. al.: Diffraction-enhanced imaging of musculoskeletal tissues using a conventional x-ray tube. Acad Radiol 2009; 16: pp. 918-923.
26. Donnelly E.F., Lewis K.G., Wolske K.M., et. al.: Characterization of the phase-contrast radiography edge-enhancement effect in a cabinet x-ray system. Phys Med Biol 2006; 51: pp. 21-30.
27. Ritman E.L.: X-ray phase-based imaging: the third wave. Acad Radiol 2009; 16: pp. 909-910.
28. Donnelly E.F., Price R.R., Lewis K.G., et. al.: Polychromatic phase-contrast computed tomography. Med Phys 2007; 34: pp. 3165-3168.
29. Pfeiffer F., Bunk O., David C., et. al.: High-resolution brain tumor visualization using three-dimensional x-ray phase tomography. Phys Med Biol 2007; 52: pp. 6923-6930.
30. Devesa I., Ferrándiz M.L., Terencio M.C., et. al.: Influence of heme oxygenase 1 modulation on the progression of murine collagen-induced arthritis. Arthritis Rheum 2005; 52: pp. 3230-3238.
31. Noyes F.R., Stabler C.L.: A system for grading articular cartilage lesions at arthroscopy. Am J Sports Med 1989; 17: pp. 505-513.
![Figure 1, ( a ) Phase-contrast computed tomographic image of the acetyl phantom at 7× magnification. The phase-contrast effect at the edge of the acetyl phantom is high for the brightness level and low for the darkness level on a gray value profile. ( b ) Gray value profiles at the edge range at 7× magnification ×7. The figure shows approximately 200 (arbitrary units [AU]) of the value of the phase-contrast effect at the edge.](https://storage.googleapis.com/dl.dentistrykey.com/clinical/ArticularCartilageImagingbytheUseofPhaseContrastTomographyinaCollagenInducedArthritisMouseModel/0_1s20S1076633209005716.jpg)