Rationale and Objectives
The purpose of this study was to assess if the presence of information including the pretest probability (Wells score), other known risk factors, and symptoms given on referrals for computed tomography (CT) pulmonary angiography correlated with prevalence rates for pulmonary embolism (PE). Also, to evaluate for differences between a university and a regional hospital setting regarding patient characteristics, amount of relevant information provided on referrals, and prevalence rates for pulmonary embolism.
Materials and Methods
Retrospective review of all consecutive referrals (emergency room, inpatient, and outpatient) for CT performed on children and adults for suspected PE from two sites: a tertiary (university) hospital (site 1) and a secondary (regional) hospital (site 2) over a 5-year period.
Results
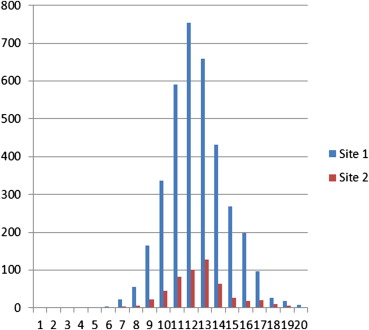
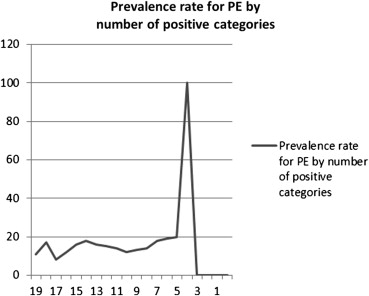
The overall prevalence rate was 510/3641 or14% of all referrals. Significantly higher number of males had a positive CT compared to women (18% versus 12%, P < .001). Although no statistically significant relationship between a greater amount of relevant information on the referral and the probability for positive finding existed, a slight trend was noted ( P = .09). In two categories, “hypoxia” and “signs of deep vein thrombosis,” the presence of this information conferred a higher probability for pulmonary embolism, P < .001. In the categories, “chest pain,” “malaise,” and “smoker/chronic obstructive pulmonary disease”, the absence of information conferred a higher probability for pulmonary embolism.
Conclusions
The amount of relevant clinical information on the request did not correlate with prevalence rates, which may reflect a lack of documentation on the part of emergency physicians who may use a “gestalt” approach. Request forms likely did not capture all relevant patient risks and many factors may interact with each other, both positively and negatively. Pretest probability estimations were rarely performed, despite their inclusion in major society guidelines.
Pulmonary embolism (PE) is a potentially life-threatening condition with a mortality rate of up to 10% in symptomatic patients within an hour of presentation and an overall mortality of 30–35% if untreated, which drops to 7% with treatment . In Sweden, 4000 patients are diagnosed with PE each year, but it is likely that many more PE go undiscovered . Data from autopsy studies estimate that only 20–30% of PE is diagnosed ante mortem . In addition, published studies demonstrate incidence rates for PE of about 23–69 per 100,000 of the population . Furthermore, only about 10% of computed tomography (CT) pulmonary angiograms (CTPAs) performed because of suspected PE are positive despite the high diagnostic accuracy (sensitivity and specificity) of CTPA . These low prevalence rates may reflect the nonspecific clinical symptoms and signs, which frequently overlap with other entities such as pneumonia and aortic and cardiac disease . The classic triad of PE symptoms—hemoptysis, shortness of breath, and chest pain—is found in less than 20% of patients with PE . In addition, most patients that present with frequent but nonspecific symptoms such as dizziness and malaise do not have PE. Therefore, a mere evaluation of symptoms and signs is not sufficient to guide selection of the appropriate imaging strategy. Many clinical decision or prediction rules exist for estimating the pretest probability of PE, including the Wells score, the Geneva Score, and the Miniati or Pisa score . There are also several recently published major society guidelines, including the European Society of Cardiology, the Fleischner Society, and the Prospective Investigation for the Diagnosis of Pulmonary Embolism investigators recommendations . Not only are clinical prediction rules and diagnostic algorithms helpful, it has even been shown that the lack of a validated diagnostic algorithm in the emergency department is an independent risk factor for inappropriate management of patients with a suspected PE . The most widely used clinical prediction rule is the Wells score, a series of criteria that in large studies have been proven to correlate with the probability of PE, the higher the given points . Factors taken into account include previous venous thromboembolism (VTE), recent surgery or immobilization, cancer, hemoptysis, tachycardia (>100 beats/min), clinical signs of deep venous thrombosis (DVT), and an alternative diagnosis less likely than PE. Scoring according to this will result in a clinical probability at two or three levels, depending on what specific version of the Wells score is used .
When the clinical probability according to the Wells score is “low/intermediate” or “PE is unlikely,” the recommendation is to proceed with a high quality d -dimer test and if the d -dimer is “negative,” PE is highly unlikely . CTPA is regarded as the diagnostic test of choice for suspected PE in most patients . Chest pain is the most frequent clinical presentation in the emergency department and in inpatients; PE is a serious diagnosis that shouldn’t be missed because the morbidity and mortality rates rise rapidly if untreated. Therefore a large number of CTPA examinations are performed to exclude PE, often in young patients, or patients in their childbearing years . Although it is important to make an accurate diagnosis early, imaging with CTPA confers several risks, which include those from iodinated contrast material, ionizing radiation to the breast tissues, chest, and the gonads (if indirect venography of the pelvis and thighs is performed) as well as the downstream effects of incidental findings . A large proportion of patients imaged with CTPA are women of childbearing age, which raises concerns for radiation exposure in these radiosensitive populations .
Get Radiology Tree app to read full this article<
Materials and methods
Study Participants
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Statistical Analysis
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Results
Get Radiology Tree app to read full this article<
Table 1
Demographics of the Patients from Both Sites
Demographic Characteristic Site 1 Site 2P Value Overall prevalence rate (%) 510 (14%) 39 (11%) NS Men (%) 1529 (42%) 219 (41%) NS Mean age men (range) 62 (0–99) 66 (0–95) <.001 Mean age women (range) 59 (1–101) 64 (16–94) <.001
NS, nonsignificant; site 1, tertiary (university) hospital; site 2, secondary (regional) hospital.
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Table 2
Relationships Concerning Probability for PE When Certain Information is Present or Absent
Category PEs Among Referrals with Denoted Information Present PEs Among Referrals with Denoted Information absent_P_ Value Hypoxia 335/2022 266/2154 <.001 † Shortness of breath 476/3226 125/950 .227 Cough 68/626 533/3550 .006 Chest pain 250/2004 351/2172 <.001 ∗ Fever 108/826 493/3350 .245 Tachycardia 204/1359 397/2817 .452 Dizziness 16/131 586/4045 .378 Malaise 128/1124 473/3052 .001 ∗ Pleuritic chest pain 121/810 480/3366 .616 D-dimer 103/678 498/3498 .473 Signs of DVT 112/488 489/3688 <.001 † Malignant disease 492/3473 109/703 .377 Operation/Immobilization 494/3474 107/702 .480 History of thrombosis 408/2794 193/1382 .606 Coagulation defects 79/481 522/3695 .189 Hormone treatment/pregnancy 22/242 579/3934 .014 Other illness 535/3661 66/515 .314 Smoker/COPD 73/746 528/3430 <.001 ∗
COPD, chronic obstructive pulmonary disease; DVT, deep vein thrombosis; PE, pulmonary embolism.
Significant relationships measured as P < .001.
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Discussion
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Conclusion
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Acknowledgments
Get Radiology Tree app to read full this article<
References
1. Dalen J.E.: Pulmonary embolism: what have we learned since Virchow? Natural history, pathophysiology, and diagnosis. Chest 2002; 122: pp. 1440-1456.
2. Dalen J.E.: Pulmonary embolism: what have we learned since Virchow? Treatment and prevention. Chest 2002; 122: pp. 1801-1817.
3. Schulman S, Lindström K. Venos thromboembolism och medel mot trombos. Available at: http://www.apoteketfarmaci.se/NyheterOchFakta/Farmaci%20Lkemedelsboken/Veno%C2%A6%C3%AAs%20tromboembolism%20och%20medel%20mot%20trombos.pdf . Accessed August 28, 2012.
4. Konstantinides S.: Clinical practice. Acute pulmonary embolism. N Engl J Med 2008; 359: pp. 2804-2813.
5. Remy Jardin M., Pistolesi M., Goodman L.R., et. al.: Management of suspected acute pulmonary embolism in the era of CT angiography: a statement from the Fleischner Society. Radiology 2007; 245: pp. 315-329.
6. Donohoo J.H., Mayo-Smith W.W., Pezzullo J.A., et. al.: Utilization patterns and diagnostic yield of 3421 consecutive multidetector row computed tomography pulmonary angiograms in a busy emergency department. J Comput Assist Tomogr 2008; 32: pp. 421-425.
7. Tapson V.F.: Advances in the diagnosis and treatment of acute pulmonary embolism. F1000 Med Rep 2012; 4: pp. 9.
8. Ouellette DR, Harrington A, Kamangar N, et al. Pulmonary embolism. Available at: http://emedicine.medscape.com/article/300901-overview . Accessed August 25, 2012.
9. Wells P.S., Anderson D.R., Rodger M., et. al.: Derivation of a simple clinical model to categorize patients probability of pulmonary embolism: increasing the models utility with the SimpliRED D-dimer. Thromb Haemost 2000; 83: pp. 416-420.
10. Le Gal G., Righini M., Roy P.M., et. al.: Prediction of pulmonary embolism in the emergency department: the revised Geneva score. Ann Intern Med 2006; 144: pp. 165-171.
11. Miniati M., Pistolesi M.: Assessing the clinical probability of pulmonary embolism. Q J Nucl Med 2001; 45: pp. 287-293.
12. Torbicki A., Perrier A., Konstantinides S., et. al., ESC Committee for Practice Guidelines (CPG): Guidelines on the diagnosis and management of acute pulmonary embolism: the Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC). Eur Heart J 2008; 29: pp. 2276-2315.
13. Stein P.D., Woodard P.K., Weg J.G., et. al., PIOPED II Investigators: Diagnostic pathways in acute pulmonary embolism: recommendations of the PIOPED II Investigators. Radiology 2007; 242: pp. 15-21.
14. Stein P.D., Sostman H.D., Bounameaux H., et. al.: Challenges in the diagnosis of acute pulmonary embolism. Am J Med 2008; 121: pp. 565-571.
15. Roy P.M., Meyer G., Vielle B., et. al.: Appropriateness of diagnostic management and outcomes of suspected pulmonary embolism. Ann Intern Med 2006; 144: pp. 157-164.
16. Gibson N.S., Sohne M., Kruip M.J., et. al.: Further validation and simplification of the Wells clinical decision rule in pulmonary embolism. Thromb Haemost 2008; 99: pp. 229-234.
17. Bajc M, Bertström O, Elf J, et al. Venös tromboembolism, Vårdprogram för södra sjukvårdsregionen. Available at: http://www.skane.se/pages/262128/V%c3%a5rdprogram%20VTE%20090629.pdf . Accessed June 2, 2013.
18. Ghaye B., Dondelinger R.F.: When to perform CTA in patients suspected of PE?. Eur Radiol 2008; 18: pp. 500-509.
19. Jha S., Ho A., Bhargavan M., et. al.: Imaging evaluation for suspected pulmonary embolism: what do emergency physicians and radiologists say?. Am J Roentgenol 2010; 194: pp. W38-W48.
20. Weiss C.R., Scatarige J.C., Diette G.B., et. al.: CT pulmonary angiography is the first-line imaging test for acute pulmonary embolism: a survey of US clinicians. Acad Radiol 2006; 13: pp. 434-446.
21. Sadigh G., Kelly A.M., Cronin P.: Challenges, controversies, and hot topics in pulmonary embolism imaging. AJR Am J Roentgenol 2011; 196: pp. 497-515.
22. Stein P.D., Fowler S.E., Goodman L.R., et. al.: Multidetector computed tomography for acute pulmonary embolism. N Engl J Med 2006; 354: pp. 2317-2327.
23. Kalva S.P., Jagannathan J.P., Hahn P.F., et. al.: Venous thromboembolism: indirect CT venography during CT pulmonary angiography–should the pelvis be imaged?. Radiology 2008; 246: pp. 605-611.
24. Parker M.S., Hui F.K., Camacho M.A., et. al.: Female breast radiation exposure during CT pulmonary angiography. AJR Am J Roentgenol 2005; 185: pp. 1228-1233.
25. Value of ventilation/perfusion scan in acute pulmonary embolism. Results of the prospective investigation of pulmonary embolism diagnosis (PIOPED). The PIOPED Investigators. JAMA 1990; 263: pp. 2753-2759.
26. Mamlouk M.D., vonSonnenberg E., Gosalia R., et. al.: Pulmonary embolism at CT angiography: implications for appropriateness, cost, and radiation exposure in 2003 patients. Radiology 2010; 256: pp. 625-632.
27. Stein P.D., Matta F.: Acute pulmonary embolism. Curr Probl Cardiol 2010; 35: pp. 314-376.
28. Parent F., Maitre S., Meyer G., et. al.: Diagnostic value of D-dimer in patients with suspected pulmonary embolism: results from a multicentre outcome study. Thromb Res 2007; 120: pp. 195-200.
29. Deonarine P., de Wet C., McGhee A.: Computed tomographic pulmonary angiography and pulmonary embolism: predictive value of a d-dimer assay. BMC Res Notes 2012; 5: pp. 104.
30. Lucassen W., Geersting G.J., Erkens P.M., et. al.: Clinical decision rules for excluding pulmonary embolism: a meta-analysis. Ann Intern Med 2011; 15587: pp. 448-460.
31. Sodickson A., Baeyens P.F., Andriole K.P., et. al.: Recurrent CT, cumulative radiation exposure, and associated radiation-induced cancer risks from CT of adults. Radiology 2009; 251: pp. 175-184.
32. Courtney D.M., Kline J.A., Kabrhel C., et. al.: Clinical features from the history and physical examination that predict the presence or absence of pulmonary embolism in symptomatic emergency department patients: results of a prospective, multicenter study. Ann Emerg Med 2010; 55: pp. 307-315.e1.
33. Drescher F.S., Chandrika S., Weir I.D., et. al.: Effectiveness and acceptability of a computerized decision support system using modified Wells criteria for evaluation of suspected pulmonary embolism. Ann Emerg Med 2011; 57: pp. 613-621.
34. Raja A.S., Ip I.K., Prevedello L.M., et. al.: Effect of computerized clinical decision support on the use and yield of CT pulmonary angiography in the emergency department. Radiology 2012; 262: pp. 468-474.