Rapid growth in the amount of data that is electronically recorded as part of routine clinical operations has generated great interest in the use of Big Data methodologies to address clinical and research questions. These methods can efficiently analyze and deliver insights from high-volume, high-variety, and high-growth rate datasets generated across the continuum of care, thereby forgoing the time, cost, and effort of more focused and controlled hypothesis-driven research. By virtue of an existing robust information technology infrastructure and years of archived digital data, radiology departments are particularly well positioned to take advantage of emerging Big Data techniques. In this review, we describe four areas in which Big Data is poised to have an immediate impact on radiology practice, research, and operations. In addition, we provide an overview of the Big Data adoption cycle and describe how academic radiology departments can promote Big Data development.
Introduction
Advances in medicine have traditionally been the result of hypothesis-driven research, often in the form of controlled clinical trials. In this approach, a clinical variable believed to influence outcome is identified a priori, and great effort is made—through patient selection and predefined research protocols—to control confounding clinical variables and isolate the effect of the variable of interest. Although this approach is effective, it may be impractical, time-consuming, and costly to run such controlled trials for each of the countless variations in patient demographics, pathophysiology, and clinical decision-making that define each case. As a result, many investigators see promise in a data-driven approach in which care is allowed to proceed as it does in the real world, and naturally occurring variations in care delivery from patient to patient are studied in aggregate to determine the effect of each on overall outcome .
This type of research relies on analytical methods from the emerging science of “Big Data” informatics. Big Data refers to extremely complex datasets characterized by the four Vs: volume , which refers to the sheer number of data elements within these extremely large datasets; variety , which describes the aggregation of data from multiple sources; velocity , which refers to the high speed at which data is generated; and veracity , which describes the inherent uncertainty in some data elements . These sources of complexity exceed the capabilities of conventional data analysis techniques, but Big Data methods are specifically designed to overcome these challenges.
This approach is inspired in part by the successes of Big Data methods in leveraging the immense data collected by mobile and internet-enabled technologies over the last decade. These data have been successfully used as the basis for targeted advertising, personalized consumer recommendations, and real-time traffic maps, among countless other applications. As electronic medical records (EMRs) and other clinical databases make patient data more readily accessible in the healthcare enterprise , there is hope that Big Data analytics may yield important insights in medicine. This vision of the future has been formalized in the concept of a Learning Healthcare System proposed by the Institute of Medicine . Indeed, early applications of Big Data to health care—such as an informatics platform to integrate neonatal physiological monitoring to predict the onset of nosocomial infections prior to the onset of clinical symptoms —have produced promising results.
The promise of Big Data is particularly strong within radiology. Nearly two decades ago, the specialty became an early adopter of digital workflows and electronic integration of healthcare information and now enjoys a mature information technology (IT) infrastructure that has virtually eradicated the use of nondigitized data . As a result, information has become the currency of radiology, and electronically accessible information—the key ingredient needed to power Big Data analytics—is available in immense quantities within the information systems at the center of every modern radiology department. Despite the rich troves of digital data available in radiology, most of the methods needed to analyze these data need to be studied and developed before the impact of Big Data on clinical radiology can be fully appreciated.
In this paper, we review potential applications of Big Data in modern radiology practice through the lens of four big questions facing our specialty. Specifically, we consider how emerging Big Data methods can enable personalized image interpretation, facilitate discovery of new imaging markers, quantify the value of radiology services to patient health, and characterize and optimize radiology workflows. We then review the four stages of Big Data adoption and use these insights as a guide for academic radiology departments that wish to encourage Big Data research, development, and utilization. In so doing, we hope to provide both inspiration and a blueprint for departmental decision-makers as the specialty of radiology steps into the next era of informatics and data science.
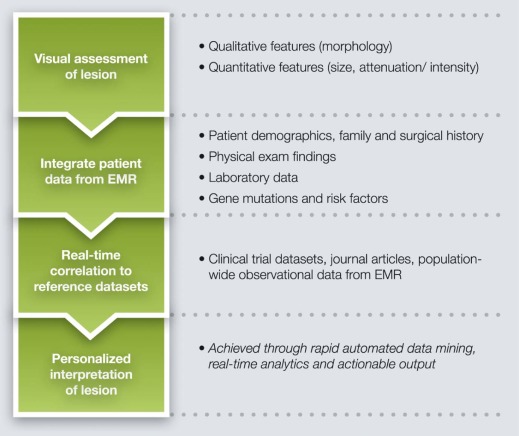
Can Image Interpretation and Management Recommendations be Personalized for Individual Patients?
Background
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Why This Needs Big Data?
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Example
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
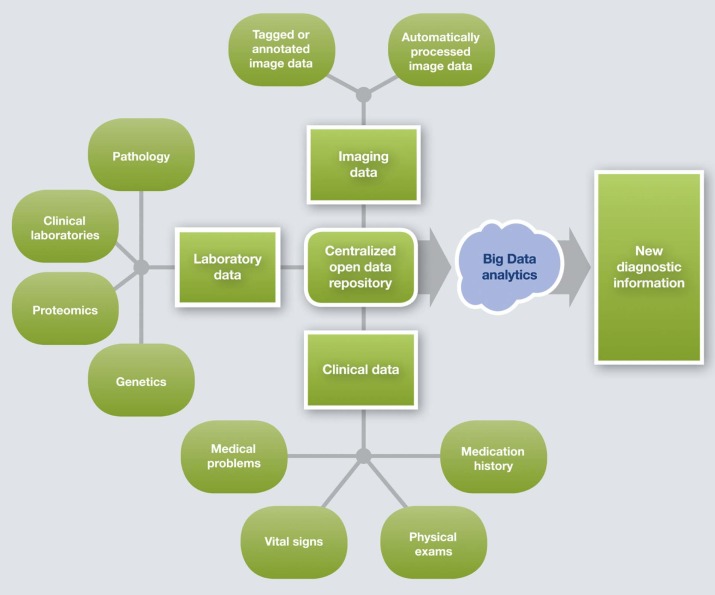
Can We Identify New Diagnostic Information in Imaging Data?
Background
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Why This Needs Big Data?
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Example
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
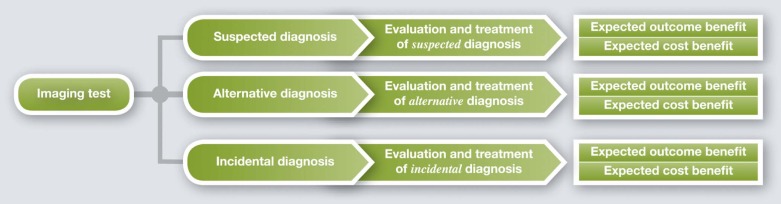
What is the Value of an Imaging Study in Terms of Clinical Outcomes and Cost of Care?
Background
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Why This Needs Big Data?
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Example
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
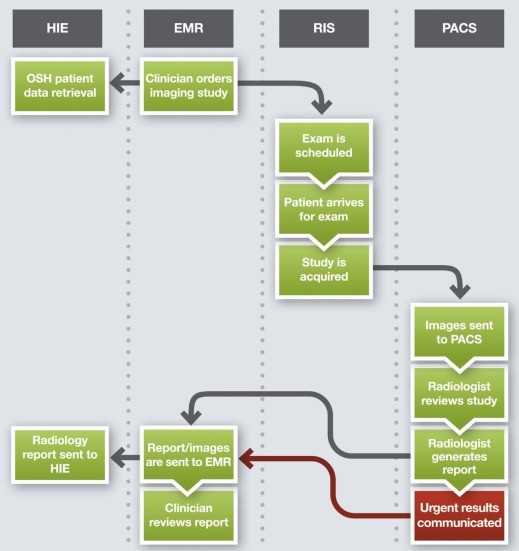
How Can We Assess and Optimize Radiology Workflows?
Background
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Why This Needs Big Data?
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Examples
Radiologist, Technologist, and Departmental Efficiency
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Diagnostic Algorithms
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Peer Review
Get Radiology Tree app to read full this article<
Cultivating Big Data Development in Radiology
Big Data Adoption Cycle
Get Radiology Tree app to read full this article<
Advancing Big Data Adoption in Radiology
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Enabling Technologies for the Next Stages
Get Radiology Tree app to read full this article<
Data Storage, Security, and Integration
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Data Extraction
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Enabling People for the Next Stage
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Barriers and Limitations
Get Radiology Tree app to read full this article<
Patient Privacy and Other Obstacles to Data Sharing
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Sparsity of High-dimensionality Data
Get Radiology Tree app to read full this article<
Statistical Challenges
Get Radiology Tree app to read full this article<
Inferior Quality of Source Data
Get Radiology Tree app to read full this article<
Conclusion
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Acknowledgment
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
References
1. Murdoch T.B., Detsky A.S.: The inevitable application of big data to health care. JAMA 2013; 309: pp. 1351-1352.
2. Badawi O., Brennan T., Celi L.A., et. al.: Making big data useful for health care: a summary of the inaugural MIT critical data conference. JMIR Med Inform 2014; 2: pp. e22.
3. McAfee A., Brynjolfsson E.: Big Data: The Management Revolution. Harvard Business Review2012.
4. Schroeck M., Schockley R.: Analytics: The real-world use of big data. Available at: http://www-935.ibm.com/services/us/gbs/thoughtleadership/ibv-big-data-at-work.html Accessed June 3, 2015
5. Groves P., Kayyali B., Knott D., et. al.: McKinsey report “The Big Data Revolution in Healthcare.”. Available at: http://www.mckinsey.com/insights/health_systems_and_services/the_big-data_revolution_in_us_health_care Accessed June 3, 2015
6. Olsen L., Aisner D., McGinnis J.M., et. al.: Summary.The learning healthcare system: workshop summary.2007.National Academies PressWashington, DC:pp. 1-36.
7. Blount M., Ebling M.R., Eklund J.M., et. al.: Real-time analysis for intensive care: development and deployment of the Artemis analytic system. IEEE Eng Med Biol Mag 2010; 29: pp. 110-118.
8. Avrin D.E., Urbania T.H.: Demise of film. Acad Radiol 2014; 21: pp. 303-304.
9. Levine D., Brown D.L., Andreotti R.F., et. al.: Management of asymptomatic ovarian and other adnexal cysts imaged at US: Society of Radiologists in Ultrasound Consensus Conference Statement. Radiology 2010; 256: pp. 943-954.
10. Mainiero M.B., Lourenco A., Mahoney M.C., et. al.: ACR appropriateness criteria breast cancer screening. J Am Coll Radiol 2013; 10: pp. 11-14.
11. Walker C., Haylock B., Husband D., et. al.: Clinical use of genotype to predict chemosensitivity in oligodendroglial tumors. Neurology 2006; 66: pp. 1661-1667.
12. Paez J.G., Janne P.A., Lee J.C., et. al.: EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 2004; 304: pp. 1497-1500.
13. Dawood S., Broglio K., Buzdar A.U., et. al.: Prognosis of women with metastatic breast cancer by HER2 status and trastuzumab treatment: an institutional-based review. J Clin Oncol 2010; 28: pp. 92-98.
14. Dilsizian S.E., Siegel E.L.: Artificial intelligence in medicine and cardiac imaging: harnessing big data and advanced computing to provide personalized medical diagnosis and treatment. Curr Cardiol Rep 2014; 16: pp. 441.
15. Howlader N., Noone A.M., Krapcho M., et. al.: SEER Cancer Statistics Review, 1975-2012. Bethesda, MD: National Cancer Institute; Available at: http://seer.cancer.gov/csr/1975_2012/ Accessed June 3, 2015
16. MacMahon H., Austin J.H., Gamsu G., et. al.: Guidelines for management of small pulmonary nodules detected on CT scans: a statement from the Fleischner Society. Radiology 2005; 237: pp. 395-400.
17. Morrison J.J., Hostetter J., Wang K., et. al.: Data-driven decision support for radiologists: re-using the National Lung Screening Trial dataset for pulmonary nodule management. J Digit Imaging 2015; 28: pp. 18-23.
18. McClelland R.L., Nasir K., Budoff M., et. al.: Arterial age as a function of coronary artery calcium (from the Multi-Ethnic Study of Atherosclerosis [MESA]). Am J Cardiol 2009; 103: pp. 59-63.
19. Malin J.L.: Envisioning Watson as a rapid-learning system for oncology. J Oncol Pract 2013; 9: pp. 155-157.
20. Pyra T., Hui B., Hanstock C., et. al.: Combined structural and neurochemical evaluation of the corticospinal tract in amyotrophic lateral sclerosis. Amyotroph Lateral Scler 2010; 11: pp. 157-165.
21. Landry G.J., Liu-Ambrose T.: Buying time: a rationale for examining the use of circadian rhythm and sleep interventions to delay progression of mild cognitive impairment to Alzheimer’s disease. Front Aging Neurosci 2014; 6: pp. 325.
22. Hebert L.E., Weuve J., Scherr P.A., et. al.: Alzheimer disease in the United States (2010-2050) estimated using the 2010 census. Neurology 2013; 80: pp. 1778-1783.
23. Langa K.M., Levine D.A.: The diagnosis and management of mild cognitive impairment: a clinical review. JAMA 2014; 312: pp. 2551-2561.
24. McEvoy L.K., Holland D., Hagler D.J., et. al.: Mild cognitive impairment: baseline and longitudinal structural MR imaging measures improve predictive prognosis. Radiology 2011; 259: pp. 834-843.
25. Kumar V., Gu Y., Basu S., et. al.: Radiomics: the process and the challenges. Magn Reson Imaging 2012; 30: pp. 1234-1248.
26. Lambin P., Rios-Velazquez E., Leijenaar R., et. al.: Radiomics: extracting more information from medical images using advanced feature analysis. Eur J Cancer 2012; 48: pp. 441-446.
27. Aerts H.J., Velazquez E.R., Leijenaar R.T., et. al.: Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat Commun 2014; 5: pp. 4006.
28. Mazurowski M.A.: Radiogenomics: what it is and why it is important. J Am Coll Radiol 2015; 12: pp. 862-866.
29. Rosenstein B.S., West C.M., Bentzen S.M., et. al.: Radiogenomics: radiobiology enters the era of big data and team science. Int J Radiat Oncol Biol Phys 2014; 89: pp. 709-713.
30. Porter M.E.: A strategy for health care reform—toward a value-based system. N Engl J Med 2009; 361: pp. 109-112.
31. Porter M.E.: What is value in health care?. N Engl J Med 2010; 363: pp. 2477-2481.
32. Bossuyt P.M., Reitsma J.B., Linnet K., et. al.: Beyond diagnostic accuracy: the clinical utility of diagnostic tests. Clin Chem 2012; 58: pp. 1636-1643.
33. Ferrante di Ruffano L., Hyde C.J., McCaffery K.J., et. al.: Assessing the value of diagnostic tests: a framework for designing and evaluating trials. BMJ 2012; 344: pp. e686.
34. Heit J., Cohen A., Anderson F.J.: Estimated annual number of incident and recurrent, non-fatal and fatal venous thromboembolism (VTE) events in the US. Blood 2005; 106: pp. 267A.
35. LaMori J.C., Shoheiber O., Mody S.H., et. al.: Inpatient resource use and cost burden of deep vein thrombosis and pulmonary embolism in the United States. Clin Ther 2015; 37: pp. 62-70.
36. Remy-Jardin M., Remy J., Wattinne L., et. al.: Central pulmonary thromboembolism: diagnosis with spiral volumetric CT with the single-breath-hold technique—comparison with pulmonary angiography. Radiology 1992; 185: pp. 381-387.
37. Anderson D.R., Kovacs M.J., Dennie C., et. al.: Use of spiral computed tomography contrast angiography and ultrasonography to exclude the diagnosis of pulmonary embolism in the emergency department. J Emerg Med 2005; 29: pp. 399-404.
38. Bettmann M.A., Baginski S.G., White R.D., et. al.: ACR Appropriateness Criteria(R) acute chest pain—suspected pulmonary embolism. J Thorac Imaging 2012; 27: pp. W28-W31.
39. Costantino M.M., Randall G., Gosselin M., et. al.: CT angiography in the evaluation of acute pulmonary embolus. AJR Am J Roentgenol 2008; 191: pp. 471-474.
40. Wiener R.S., Schwartz L.M., Woloshin S.: Time trends in pulmonary embolism in the United States: evidence of overdiagnosis. Arch Intern Med 2011; 171: pp. 831-837.
41. Khorasani R.: You should eliminate paper from your PACS workflow: why and how?. J Am Coll Radiol 2006; 3: pp. 628-629.
42. Prevedello L., Khorasani R.: Which radiology application drives your workflow? Which should?. J Am Coll Radiol 2013; 10: pp. 80-82.
43. Nagy P.G., Warnock M.J., Daly M., et. al.: Informatics in radiology: automated Web-based graphical dashboard for radiology operational business intelligence. Radiographics 2009; 29: pp. 1897-1906.
44. Lu L., Li J., Gisler P.: Improving financial performance by modeling and analysis of radiology procedure scheduling at a large community hospital. J Med Syst 2011; 35: pp. 299-307.
45. Lakhani P., Kim W., Langlotz C.P.: Automated detection of critical results in radiology reports. J Digit Imaging 2012; 25: pp. 30-36.
46. Bhargavan M., Sunshine J.H.: Utilization of radiology services in the United States: levels and trends in modalities, regions, and populations. Radiology 2005; 234: pp. 824-832.
47. Parker L., Levin D.C., Frangos A., et. al.: Geographic variation in the utilization of noninvasive diagnostic imaging: national medicare data, 1998-2007. AJR Am J Roentgenol 2010; 194: pp. 1034-1039.
48. Patti J.A.: The national radiology data registry: a necessary component of quality health care. J Am Coll Radiol 2011; 8: pp. 453.
49. Rosen M.P., Ding A., Blake M.A., et. al.: ACR Appropriateness Criteria(R) right lower quadrant pain—suspected appendicitis. J Am Coll Radiol 2011; 8: pp. 749-755.
50. Trout A.T., Sanchez R., Ladino-Torres M.F., et. al.: A critical evaluation of US for the diagnosis of pediatric acute appendicitis in a real-life setting: how can we improve the diagnostic value of sonography?. Pediatr Radiol 2012; 42: pp. 813-823.
51. Abujudeh H., Pyatt R.S., Bruno M.A., et. al.: RADPEER peer review: relevance, use, concerns, challenges, and direction forward. J Am Coll Radiol 2014; 11: pp. 899-904.
52. Strowig G.: Making the switch to vendor-neutral archiving. How to optimize comprehensive data storage for healthcare’s new age. Health Manag Technol 2013; 34: pp. 10. 2-3
53. Puppala M., He T., Chen S., et. al.: METEOR: an enterprise health informatics environment to support evidence-based medicine. IEEE Trans Biomed Eng 2015; http://dx.doi.org/10.1109/TBME.2015.2450181
54. Starren J.B., Winter A.Q., Lloyd-Jones D.M.: Enabling a learning health system through a unified enterprise data warehouse: the experience of the Northwestern University Clinical and Translational Sciences (NUCATS) Institute. Clin Transl Sci 2015; 8: pp. 269-271.
55. Evans R.S., Lloyd J.F., Pierce L.A.: Clinical use of an enterprise data warehouse. AMIA Annu Symp Proc 2012; 2012: pp. 189-198.
56. Horvath M.M., Winfield S., Evans S., et. al.: The DEDUCE Guided Query tool: providing simplified access to clinical data for research and quality improvement. J Biomed Inform 2011; 44: pp. 266-276.
57. Chute C.G., Beck S.A., Fisk T.B., et. al.: The enterprise data trust at Mayo Clinic: a semantically integrated warehouse of biomedical data. J Am Med Inform Assoc 2010; 17: pp. 131-135.
58. Lacson R., Khorasani R.: Practical examples of natural language processing in radiology. J Am Coll Radiol 2011; 8: pp. 872-874.
59. Channin D.S., Mongkolwat P., Kleper V., et. al.: The annotation and image mark-up project. Radiology 2009; 253: pp. 590-592.
60. Reiner B.I.: Creating accountability in image quality analysis part 3: creation of a standardized image-centric mark-up and annotation tool. J Digit Imaging 2013; 26: pp. 600-604.
61. Gorgolewski K., Burns C.D., Madison C., et. al.: Nipype: a flexible, lightweight and extensible neuroimaging data processing framework in python. Front Neuroinform 2011; 5: pp. 13.
62. Zijdenbos A.P., Forghani R., Evans A.C.: Automatic “pipeline” analysis of 3-D MRI data for clinical trials: application to multiple sclerosis. IEEE Trans Med Imaging 2002; 21: pp. 1280-1291.
63. Jee K., Kim G.H.: Potentiality of big data in the medical sector: focus on how to reshape the healthcare system. Healthc Inform Res 2013; 19: pp. 79-85.
64. Mennes M., Biswal B.B., Castellanos F.X., et. al.: Making data sharing work: the FCP/INDI experience. Neuroimage 2013; 82: pp. 683-691.
65. Robson B.: The dragon on the gold: myths and realities for data mining in biomedicine and biotechnology using digital and molecular libraries. J Proteome Res 2004; 3: pp. 1113-1119.
66. Sox H.C., Goodman S.N.: The methods of comparative effectiveness research. Annu Rev Public Health 2012; 33: pp. 425-445.
67. Grossmann C., Powers B., Sanders J., et. al.: Issues and opportunities in the emergence of large health-related datasets.Digital data improvement priorities for continuous learning in health and health care: workshop summary.2013.National Academies PressWashington, DC:pp. 27-32.
68. Olsen L., Grossmann C., McGinnis J.M., et. al.: Moving forward.Learning what works: infrastructure required for comparative effectiveness research: workshop summary.2011.National Academies PressWashington, DC:pp. 315-332.
69. Lord S.J., Gebski V.J., Keech A.C.: Multiple analyses in clinical trials: sound science or data dredging?. Med J Aust 2004; 181: pp. 452-454.