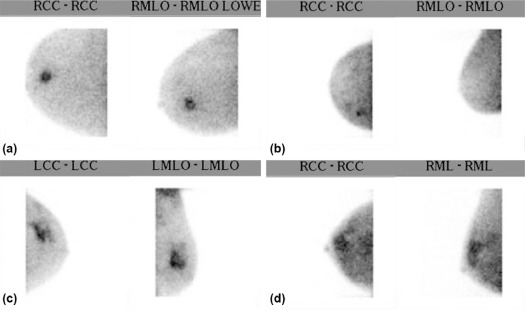
Rationale and Objectives
To evaluate correlations between molecular breast imaging (MBI) descriptor characteristics and positive predictive value (PPV) in detecting breast cancer.
Materials and Methods
A retrospective review was performed on 193 suspicious findings from 153 women (31–81 years) with positive MBI examinations. We assessed associations between (i) lesion pattern (mass vs. nonmass) and PPV; (ii) lesion pattern and suspected likelihood of cancer (low vs. moderate vs. high); (iii) background parenchymal uptake (BPU) (homogeneous vs. heterogeneous) and PPV; (iv) breast density (dense vs. non-dense) and PPV; and (v) BPU and density.
Results
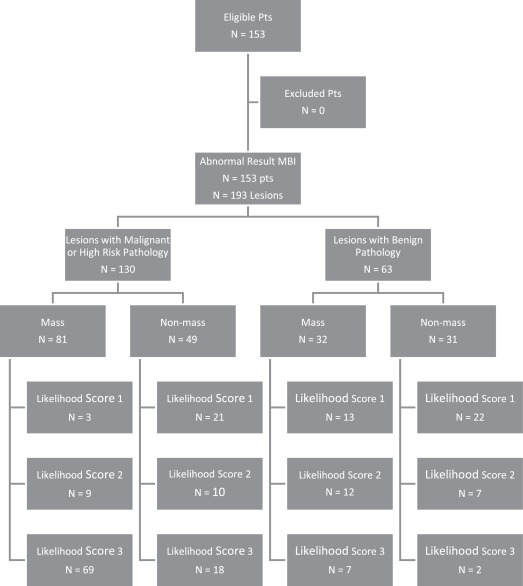
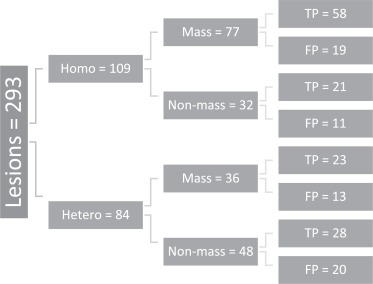
One hundred ten of 153 patients were diagnosed with malignancy or high-risk pathology (PPV1 = 71.9%), and 130/193 biopsies resulted in malignant or high-risk lesions (PPV3 = 67.4%). Biopsies of mass vs. nonmass findings had comparable PPV3 (71.7% vs. 61.3%; P = .0717). Mass findings were correlated with higher suspicion for cancer than nonmass findings ( P < .001). There was no significant difference in PPV3 when comparing biopsies from homogeneous vs. heterogeneous BPU (72.5% vs. 60.7%; P = .103). No association was found between patients’ BPU and diagnosed cancer or high-risk lesions ( P = .513). Biopsies from nondense breasts demonstrated higher PPV3 than biopsies from dense breasts (85.4% vs. 60.6%; P = .0025); patients with nondense breasts were more likely to be diagnosed with cancer or high-risk pathology (PPV1 = 87.8% vs. 66.0%; P = .00844). Dense breasts had a greater association with heterogeneous BPU ( P = .0844).
Conclusion
Neither variability in mass or nonmass positive MBI findings, nor variability in BPU on MBI were significant determinants for the probability of malignancy. Dense breasts were associated with lower predictability and heterogeneous BPU on MBI.
Introduction
Molecular breast imaging (MBI), also known as breast-specific gamma imaging, is increasingly being used as an adjunct imaging modality in the detection of breast cancer. In recent years, breast-optimized gamma detectors have been noted to reliably detect tumors less than 1 cm in size . A meta-analysis in 2013 from 8 studies, including 2183 lesions, showed that the sensitivity and specificity of MBI were 95% and 80%, respectively . In addition to demonstrating a sensitivity and specificity in diagnosing breast cancer comparable to that of magnetic resonance imaging (MRI), MBI also has many advantages for clinical use . While mammography is affected by breast density, MBI has been shown to be reliable irrespective of breast density . MBI is also a feasible screening alternative for women who refuse to undergo MRI due to claustrophobia, which may hinder up to about 25% of women, including those at high breast cancer risk .
Currently accepted clinical and research indications of MBI include, but are not limited to, the extent of disease/preoperative staging in newly diagnosed breast cancer, the evaluation of response to neoadjuvant chemotherapy, the detection of local breast cancer recurrence, the evaluation for primary breast cancer in women with metastases or metastatic axillary lymphadenopathy of unknown primary, breast cancer screening, an adjunct to conventional breast imaging for problem solving in indeterminate cases, technically difficult breast imaging, and patients for whom breast MRI would be indicated but is not possible due to renal insufficiency, implanted devices, body habitus, or claustrophobia .
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Materials and Methods
Patients
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
MBI Technique and Interpretation
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Pathologic Diagnosis and Follow-Up Correlation
Get Radiology Tree app to read full this article<
Statistical Analysis
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Results
Overview
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
TABLE 1
A—Pathologic Findings of Malignant Lesions Detected Using MBI. B—Pathologic Findings of High-Risk Lesions Detected Using MBI. C—Pathologic Findings of Benign Lesions Detected Using MBI
a Pathology of Malignant Lesions Detected Using MBI Malignant Types No. of Lesions No. of Mass No. of Nonmass Ductal carcinoma in situ 35 18 17 Invasive ductal carcinoma 24 21 3 Invasive ductal carcinoma with a component of ductal carcinoma in situ 44 31 13 Invasive lobular carcinoma 8 4 4 Total 111 74 37
b Pathology of High-Risk Lesions Detected Using MBI High-Risk Types No. of Lesions No. of Mass No. of Nonmass Atypical ductal hyperplasia 6 4 2 Atypical lobular hyperplasia 5 3 2 Lobular carcinoma in situ 3 0 3 Papillomatosis 3 0 3 Radial scar 2 0 2 Total 19 7 12
c Pathology of Benign Findings Detected Using MBI Benign Types No. of Findings No. of Mass No. of Nonmass Apocrine metaplasia 1 1 0 Benign breast tissue 8 4 4 Fibroadenoma 6 3 3 Fibrocystic change 25 12 13 Florid adenosis 2 1 1 Organizing hematoma and fat necrosis 1 1 0 Pseudoangiomatosis hyperplasia 1 1 0 Sclerosing adenosis 4 3 1 Stromal fibrosis 10 4 6 Usual ductal hyperplasia 5 2 3 Total 63 32 31
Get Radiology Tree app to read full this article<
Character of Suspicious Findings
Get Radiology Tree app to read full this article<
Likelihood of Cancer Score
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Character of BPU
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Density
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Comparison of BPU and Breast Density
Get Radiology Tree app to read full this article<
Discussion
Overall
Get Radiology Tree app to read full this article<
Character of Suspicious Findings
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Likelihood of Cancer Score
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Character of BPU
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Density
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Conclusions
Get Radiology Tree app to read full this article<
Limitations
Get Radiology Tree app to read full this article<
Acknowledgements
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
References
1. Tadwalkar R., Rapelyea J., Torrente J., et. al.: Breast-specific gamma imaging as an adjunct modality for the diagnosis of invasive breast cancer with correlation to tumour size and grade. Br J Radiol 2012; 85: pp. e212-e216.
2. Garibaldi F., Cisbani E., Cusanno F., et. al.: Optimization of compact gamma cameras for breast imaging. Nucl Instruments Methods Phys Res Sect A 2001; 471: pp. 222-228.
3. Scopinaro F., Pani R., De Vincentis G., et. al.: High-resolution scintimammography improves the accuracy of technetium-99m methoxyisobutylisonitrile scintimammography: use of a new dedicated gamma camera. Eur J Nucl Med 1999; 26: pp. 1279-1288.
4. Sun Y., Wei W., Yang H.W., et. al.: Clinical usefulness of breast-specific gamma imaging as an adjunct modality to mammography for diagnosis of breast cancer: a systemic review and meta-analysis. Eur J Nucl Med Mol Imaging 2013; 40: pp. 450-463.
5. Weigert J., Bertrand M., Lanzkowsky L., et. al.: Results of a multicenter patient registry to determine the clinical impact of breast-specific gamma imaging, a molecular breast imaging technique. Am J Roentgenol 2012; 198: pp. W69-W75.
6. Zhang A., Li P., Liu Q., et. al.: Breast-specific gamma camera imaging with 99mTc-MIBI has better diagnostic performance than magnetic resonance imaging in breast cancer patients: a meta-analysis. Hell J Nucl Med 2017; 20: pp. 26-35.
7. Brem R., Petrovitch I., Rapelyea J., et. al.: Breast-specific gamma imaging with 99mTc-sestamibi and magnetic resonance imaging in the diagnosis of breast cancer–a comparative study. Breast J 2007; 13: pp. 465-469.
8. Hruska C.: Molecular breast imaging for screening in dense breasts: state of the art and future directions. Am J Roentgenol 2017; 208: pp. 275-283.
9. Rhodes D., Hruska C., Phillips S., et. al.: Dedicated dual-head gamma imaging for breast cancer screening in women with mammographically dense breasts. Radiology 2011; 258: pp. 106-118.
10. Khalkhali I., Baum J.K., Villanueva-Meyer J., et. al.: 99mTc-sestamibi breast imaging for the examination of patients with dense and fatty breasts: multicenter study. Radiology 2002; 222: pp. 149-155.
11. Cutrone J.A., Khalkhali I., Yosper L.S., et. al.: Tc-99m sestamibi scintimammography for the evaluation of breast masses in patients with radiographically dense breasts. Breast J 1999; 5: pp. 383-388.
12. Berg W., Blume J., Mendelson E., et. al.: Reasons women at elevated risk of breast cancer refuse breast MR imaging screening: ACRIN 6666. Radiology 2010; 254: pp. 79-87.
13. ACR Practice Parameter for the Performance of Molecular Breast Imaging (MBI) Using A Dedicated Gamma Camera. The American College of Radiology2017.
14. Goldsmith S., Parsons W., Stabin M., et. al.: SNM practice guideline for breast scintigraphy with breast-specific gamma-cameras 1.0. J Nucl Med Technol 2010; 38: pp. 219-224.
15. Conners A., Hruska C., Berg W., et. al.: Lexicon for standardized interpretation of gamma camera molecular breast imaging: observer agreement and diagnostic accuracy. Eur J Nucl Med Mol Imaging 2012; 39: pp. 971-982.
16. Kuhn K., Rapelyea J., Torrente J., et. al.: Comparative diagnostic utility of low-dose breast-specific gamma imaging to current clinical standard. Breast J 2016; 22: pp. 180-188.
17. Sickles E.A., D’Orsi C.J., Bassett L.W., et. al.: ACR BI-RADS® mammography.ACR BI-RADS® atlas, Breast Imaging Reporting and Data System.2013.American College of Radiology.Reston, VA:pp. 121-140.
18. Burnside E., Sickles E., D’Orsi C., et. al.: Original article: the ACR BI-RADS® experience: learning from history. J Am Coll Radiol 2009; 6: pp. 851-860.
19. Rosenberg R.D., Hung W.C., Williamson M.R., et. al.: Effects of age, breast density, ethnicity, and estrogen replacement therapy on screening mammographic sensitivity and cancer stage at diagnosis: review of 183,134 screening mammograms in Albuquerque, New Mexico. Radiology 1998; 209: pp. 511-518.
20. Kolb T.M., Lichy J., Newhouse J.H.: Comparison of the performance of screening mammography, physical examination, and breast US and evaluation of factors that influence them: an analysis of 27,825 patient evaluations. Radiology 2002; 225: pp. 165-175.
21. Brem R., Floerke A., Mathur V., et. al.: Breast-specific gamma imaging as an adjunct imaging modality for the diagnosis of breast cancer. Radiology 2008; 247: pp. 651-657.
22. Moriguchi S.M., De Luca L.A., Griva B.L., et. al.: Accuracy of 99mTc-sestamibi scintimammography for breast cancer diagnosis. Exp Ther Med 2010; 1: pp. 205-209.
23. Conners A., Jones K., Hruska C., et. al.: Direct-conversion molecular breast imaging of invasive breast cancer: imaging features, extent of invasive disease, and comparison between invasive ductal and lobular histology. AJR Am J Roentgenol 2015; 205: pp. W374-W381.
24. Buscombe J.R., Cwikla J.B., Thakrar D.S., et. al.: Uptake of Tc-99m MIBI related to tumour size and type. Anticancer Res 1997; 17: pp. 1693-1694.
25. Meissnitzer T., Meissnitzer M.W., Seymer A., et. al.: Relative uptake factor of invasive ductal breast cancer in breast-specific gamma imaging as a surrogate parameter for sub-typing. Anticancer Res 2015; 35: pp. 5671-5677.
26. Lee S.J., Choi Y.Y., Kim C., et. al.: Correlations between tumor to background ratio on breast-specific gamma imaging and prognostic factors in breast cancer. J Korean Med Sci 2017; 32: pp. 1031-1037.
27. Van den Bosch M., Daniel B., Ikeda D., et. al.: Magnetic resonance imaging characteristics of fibrocystic change of the breast. Invest Radiol 2005; 40: pp. 436-441.
28. Chen J., Liu H., Baek H., et. al.: Original contribution: magnetic resonance imaging features of fibrocystic change of the breast. Magn Reson Imaging 2008; 26: pp. 1207-1214.
29. Schnitt S.J., Collins L.C.: Pathology of benign breast disorders.Harris J.R.Lippman M.E.Morrow M. et. al.Breast diseases.2010.Lippincottpp. 69.
30. Love S., Gelman R., Silen W.: Sounding board. Fibrocystic “disease” of the breast–a nondisease?. N Engl J Med 1982; 307: pp. 1010-1014.
31. Delmon-Moingeon L.I., Piwnica-Worms D., Van den Abbeele A.D., et. al.: Uptake of the cation hexakis (2-methoxyisobutylisonitrile)-technetium-99m by human carcinoma cell lines in vitro. Cancer Res 1990; 50: pp. 2198-2202.
32. Cordobes M.D., Starzec A., Delmon-Moingeon L., et. al.: Technetium-99m-sestamibi uptake by human benign and malignant breast tumor cells: correlation with MDR gene expression. J Nucl Med 1996; 37: pp. 286-289.
33. Hruska C., Conners A., Rhodes D., et. al.: Original investigation: effect of menstrual cycle phase on background parenchymal uptake at molecular breast imaging. Acad Radiol 2015; 22: pp. 1147-1156.
34. Hruska C., Rhodes D., Vachon C., et. al.: Background parenchymal uptake during molecular breast imaging and associated clinical factors. AJR Am J Roentgenol 2015; 204: pp. W363-W370.
35. Scopinaro F., Schillaci O., Scarpini M., et. al.: Technetium-99m sestamibi: an indicator of breast cancer invasiveness. Eur J Nucl Med 1994; 21: pp. 984-987.
36. Larson M., Li Z., Zhao M., et. al.: Physiological fluctuation of 99mTc-sestamibi uptake in normal mammary glands: a systematic investigation in female rats. Acta Radiol 2009; 50: pp. 975-978.
37. Mankoff D.A., Dunnwald L.K., Gralow J.R., et. al.: Tc-99m-sestamibi (MIBI) uptake and washout in locally advanced breast cancer (LABC) correlates with tumor blood flow. J Nucl Med 2002; 42: pp. 23P-24P.
38. Hruska C., Scott C., Vachon C., et. al.: Background parenchymal uptake on molecular breast imaging as a breast cancer risk factor: a case-control study. Breast Cancer Res 2016; 18: pp. 1-11.
39. Thomsen S., Tatman D.: Physiological and pathological factors of human breast disease that can influence optical diagnosis. Ann N Y Acad Sci 1998; 838: pp. 171-193.
40. Rechtman L.R., Lenihan M.J., Lieberman J.H., et. al.: Breast-specific gamma imaging for the detection of breast cancer in dense versus nondense breasts. Am J Roentgenol 2014; 202: pp. 293-298.
41. Brem R., Ruda R., Yang J., et. al.: Breast-specific γ-imaging for the detection of mammographically occult breast cancer in women at increased risk. J Nucl Med 2016; 57: pp. 678-684.
42. Sprague B.L., Gangnon R.E., Burt V., et. al.: Prevalence of mammographically dense breasts in the United States. J Natl Cancer Inst 2014; 106: dju255
43. Kim M.Y., Choi N., Ko S.M., et. al.: Background uptake of breast-specific gamma imaging: correlation with mammographic breast density and background enhancement of breast MRI. Clin Imaging 2014; 38: pp. 255-258.
44. Yoon H., Kim Y., Lee J., et. al.: Background 99mTc-methoxyisobutylisonitrile uptake of breast-specific gamma imaging in relation to background parenchymal enhancement in magnetic resonance imaging. Eur Radiol 2015; 25: pp. 32-40.
45. Pike M.C., Pearce C.L.: Mammographic density, MRI background parenchymal enhancement and breast cancer risk. Ann Oncol 2013; 24: pp. viii37-viii41.
46. DeMartini W.B., Liu F., Peacock S., et. al.: Background parenchymal enhancement on breast MRI: impact on diagnostic performance. Am J Roentgenol 2012; 198: pp. W373-W380.