Rationale and Objectives
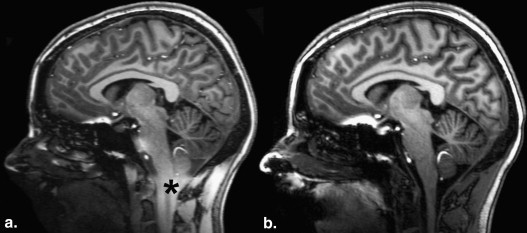
The magnetization-prepared rapid-acquisition gradient-echo (MPRAGE) sequence regularly shows caudal image contrast inversion at 7 T and therefore reduced clinical applicability. The investigators report the technical source of this problem and present a practical solution.
Materials and Methods
A total of 71 subjects were scanned using a 7-T whole-body magnetic resonance imaging system using a 32-channel transmit/receive head coil. In 39 subjects, 45 high-resolution T1 contrast image data sets were acquired with the standard MPRAGE sequence. A modified sequence with an adiabatic wideband uniform rate smooth truncation pulse for magnetization preparation was used for 45 further scans in 39 subjects. In total, seven subjects underwent scans with both sequences. The homogeneity of T1 contrast and the occurrence of caudal image contrast inversion were evaluated in consensus reading by two neuroradiologists.
Results
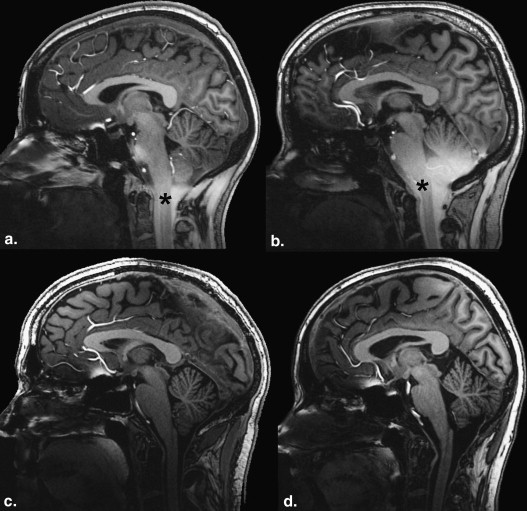
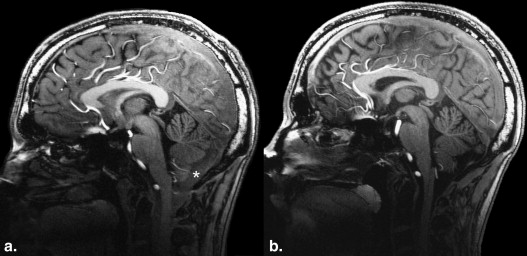
Caudal image contrast inversion was depicted in 19 acquisitions (42.2%) using the standard MPRAGE sequence. Using the adiabatic wideband uniform rate smooth truncation pulse for magnetization preparation, caudal image contrast inversion was depicted in only three acquisitions (6.7%). A χ 2 test showed a significant difference between the two preparation pulses ( P < .001).
Conclusions
Magnetization preparation with an adiabatic wideband uniform rate smooth truncation pulse in the MPRAGE sequence at 7 T can significantly reduce the occurrence of caudal image contrast inversion and improves signal homogeneity.
Ultra-high-field magnetic resonance imaging (MRI) at 7 T is currently being considered for clinical application . Many technical difficulties have been mastered already, but several issues remain to be solved. Reliability and reproducibility of the acquired images are two of the main goals that must be achieved. The magnetization-prepared rapid-acquisition gradient-echo (MPRAGE) sequence using a hyperbolic secant inversion pulse is a standard sequence for T1 contrast brain imaging at high magnetic field strengths ; unfortunately, it regularly shows caudal contrast inversion (CCI) at 7 T and therefore reduced clinical applicability.
Static magnetic field inhomogeneities, ΔB 0 , which arise for example from susceptibility changes at tissue boundaries, are responsible among other factors for off-resonance frequency content inside the field of view. At ultrahigh fields, off-resonance frequencies are generally higher because of the higher ΔB 0 , which scales linearly with field strength . Hence, shimming of the main magnetic field is very crucial to curtail B 0 inhomogeneities inside the imaging area . Because of the limited bandwidth of the conventional radiofrequency (RF) inversion pulse, tissues with large frequency shifts may not experience effective magnetization preparation, leading to unexpected contrast inversion.
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Materials and methods
Study Population
Get Radiology Tree app to read full this article<
Scanner and Coil System
Get Radiology Tree app to read full this article<
Examination at 7 T
Get Radiology Tree app to read full this article<
Adiabatic RF Pulses
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Q=[(ω)21+Δω2]32∣∣ω1(dΔωdt)−Δω(dω1dt)∣∣, Q
=
[
(
ω
)
1
2
+
Δ
ω
2
]
2
3
|
ω
1
(
d
Δ
ω
d
t
)
−
Δ
ω
(
d
ω
1
d
t
)
|
,
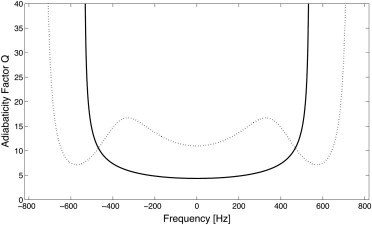
where ω 1 = γB 1 ( t ) (the RF pulse envelope times the gyromagnetic ratio γ = 42.57 MHz/T), and Δω is the frequency sweep of the adiabatic RF pulse. In principle, Q should be large compared with unity: the higher the value of Q , the lower the sensitivity to areas in which the maximal amplitude B 1 max of the pulse is not reached. Q scales with B 1 max , but for most in vivo measurements B 1 max is restricted because of specific absorption rate (SAR) limitations. In the literature, values of Q ≥ 5 have been recommended .
Get Radiology Tree app to read full this article<
MPRAGE Sequence
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Simulations
Get Radiology Tree app to read full this article<
Image Evaluation
Get Radiology Tree app to read full this article<
Results
Study Group
Get Radiology Tree app to read full this article<
Table 1
Occurrence of CCI
Hyperbolic Secant Pulse WURST Pulse No CCI 26 42 CCI 19 3
CCI, caudal contrast inversion; WURST, wideband uniform rate smooth truncation.
Rates of occurrence of CCI for the pulses used for magnetization preparation are shown. A χ 2 test showed significant difference between the two pulses ( P < .001).
Table 2
Occurrence of CCI for the Two Magnetization Preparation Pulses in Volunteers and Patients Who Underwent Multiple Scans
Subject Hyperbolic Secant Pulse WURST Pulse No CCI CCI No CCI CCI Volunteer 1 1 1 Volunteer 2 1 2 Volunteer 3 1 ∗ 1 ∗ Volunteer 4 1 2 Volunteer 5 1 2 Volunteer 6 1 ‡ 1 ‡ Volunteer 7 2 † 3 Volunteer 8 1 ∗ 2 ∗,† Patient 1 1 1 Patient 2 1 1 Patient 3 2 Patient 4 1 ∗ 1 ∗
CCI, caudal contrast inversion; WURST, wideband uniform rate smooth truncation.
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Unmodified and Modified MPRAGE Sequences
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Occurrence of CCI
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Discussion
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Acknowledgments
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
References
1. Ladd M.E.: High-field-strength magnetic resonance: potential and limits. Top Magn Reson Imaging 2007; 18: pp. 139-152.
2. Fujii Y., Uzuka T., Matsuzawa H., et. al.: Neuroscientific application of ultra high-field (7 Tesla) MRI. Neurolog Surg 2010; 38: pp. 107-116.
3. Kollia K., Maderwald S., Putzki N., et. al.: First clinical study on ultra-high-field MR imaging in patients with multiple sclerosis: comparison of 1.5T and 7T. AJNR Am J Neuroradiol 2009; 30: pp. 699-702.
4. Moenninghoff C., Maderwald S., Theysohn J.M., et. al.: Imaging of adult astrocytic brain tumours with 7 T MRI: preliminary results. Eur Radiol 2010; 20: pp. 704-713.
5. Mugler J.P., Brookeman J.R.: Three-dimensional magnetization-prepared rapid gradient-echo imaging (3D MP RAGE). Magn Reson Med 1990; 15: pp. 152-157.
6. Mugler J.P., Brookeman J.R.: Rapid three-dimensional T1-weighted MR imaging with the MP-RAGE sequence. J Magn Reson Imaging 1991; 1: pp. 561-567.
7. Van de Moortele P.F., Auerbach E.J., Olman C., et. al.: T1 weighted brain images at 7 Tesla unbiased for proton density, T2∗ contrast and RF coil receive B1 sensitivity with simultaneous vessel visualization. Neuroimage 2009; 46: pp. 432-446.
8. Hurley A.C., Al-Radaideh A., Bai L., et. al.: Tailored RF pulse for magnetization inversion at ultrahigh field. Magn Reson Med 2010; 63: pp. 51-58.
9. Chmurny G.N., Hoult D.I.: The ancient and honourable art of shimming. Concepts Magnc Reson 1990; 2: pp. 131-149.
10. Sengupta S., Welch E.B., Zhao Y., et. al.: Dynamic B0 shimming at 7 T. Magn Reson Imaging 2011; 29: pp. 483-496.
11. Pang Y., Shen G.X.: Improving excitation and inversion accuracy by optimized RF pulse using genetic algorithm. J Magn Reson 2007; 186: pp. 86-93.
12. Shen J., Chen Z., Yang J.: New FOCI pulses with reduced radiofrequency power requirements. J Magn Reson Imaging 2004; 20: pp. 531-537.
13. Yongbi M.N., Yang Y., Frank J.A., et. al.: Multislice perfusion imaging in human brain using the C-FOCI inversion pulse: comparison with hyperbolic secant. Magn Reson Med 1999; 42: pp. 1098-1105.
14. Ordidge R.J., Wylezinska M., Hugg J.W., et. al.: Frequency offset corrected inversion (FOCI) pulses for use in localized spectroscopy. Magn Reson Med 1996; 36: pp. 562-566.
15. Conolly S., Nishimura D., Macovski A., et. al.: Variable-rate selective excitation. J Magn Reson 1988; 78: pp. 440-458.
16. Kupce E., Freeman R.: Adiabatic pulses for wideband inversion and broadband decoupling. J Magn Reson Ser A 1995; 115: pp. 273-276.
17. Schar M., Kozerke S., Fischer S.E., et. al.: Cardiac SSFP imaging at 3 Tesla. Magn Reson Med 2004; 51: pp. 799-806.
18. Hoult D.I.: The Principle of reciprocity in signal strength calculations—a mathematical guide. Concepts Magn Reson 2000; 12: pp. 173-187.
19. Bernstein M.A., King K.F., Zhou Z.J.: Handbook of MRI pulse sequences.2004.Academic PressBoston, MA
20. Kupce E., Freeman R.: Stretched adiabatic pulses for broadband spin inversion. J Magn Reson Ser A 1995; 117: pp. 246-256.
21. Baum J., Tycko R., Pines A.: Broadband and adiabatic inversion of a two-level system by phase-modulated pulses. Phys Rev A 1985; 32: pp. 3435.
22. Silver M.S., Joseph R.I., Hoult D.I.: Highly selective π / 2 and π pulse generation. J Magn Reson 1984; 59: pp. 347-351.
23. Garwood M., DelaBarre L.: The return of the frequency sweep: designing adiabatic pulses for contemporary NMR. J Magn Reson 2001; 153: pp. 155-177.
24. Truong T.-K., Chakeres D.W., Beversdorf D.Q., et. al.: Effects of static and radiofrequency magnetic field inhomogeneity in ultra-high field magnetic resonance imaging. Magn Reson Imaging 2006; 24: pp. 103-112.
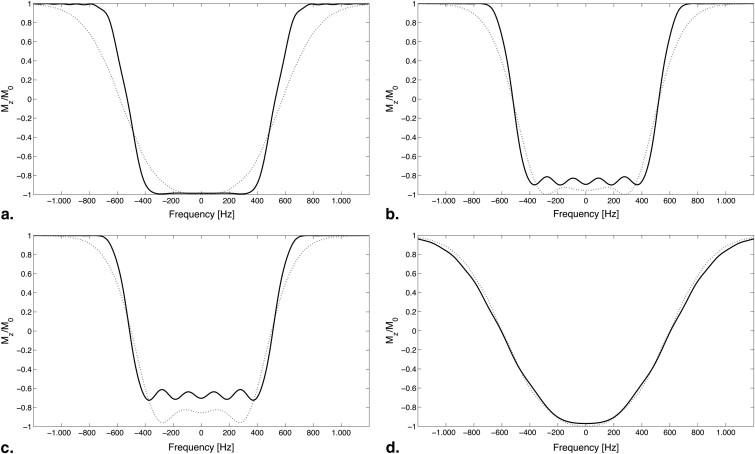
![Figure 1, B 1 envelopes (a) and frequency sweep (b) of radiofrequency (RF) pulses used for magnetization preparation ( solid line , hyperbolic secant; dotted line , wideband uniform rate smooth truncation [WURST] pulse). The duration of both pulses was 10.240 μs. The WURST pulse has a broader frequency range of ±730 Hz compared to ±535 Hz for the hyperbolic secant RF pulse.](https://storage.googleapis.com/dl.dentistrykey.com/clinical/CaudalImageContrastInversioninMPRAGEat7Tesla/0_1s20S1076633211004600.jpg)