Rationale and Objectives
To characterize practices and quantify variation in longitudinal follow-up approaches among interventional radiologists (IRs) after liver transarterial locoregional therapy (LRT) in contemporary Interventional Oncology practice.
Materials and Methods
In November/December 2014, Society of Interventional Radiology members were invited to participate in a survey regarding clinical and imaging follow-up of liver cancer patients treated with transarterial LRT. On survey closure, responses were compiled and analyzed.
Results
The 30-item survey response rate was 11% (361 of 3290). Respondents were predominantly American IRs (311 of 355, 88%) who perform 1–5 LRTs monthly (196 of 354, 55%). Most (305 of 336, 91%) IRs reported longitudinal follow-up, with patient encounters within 1-month (73%, 211 of 290) postprocedure and every 3 months (68%, 196 of 287) thereafter and involvement in imaging (up to 80%, 235 of 290) ordering and evaluation. Preferred timing of first follow-up imaging (1 month vs. 3 months) and response criteria used (mRECIST favored) varied.
Conclusions
Although IRs are actively involved in clinical and imaging follow-up of patients with liver malignancies treated with transarterial LRTs, there are differences in imaging frequency and response assessment. These data may serve as a starting point for standardization of LRT follow-up.
Longitudinal care of Interventional Oncology (IO) patients may have an important role for optimizing clinical outcomes and is critical for the acceptance of Interventional Radiologists (IRs) into the cancer care treatment paradigm. Although essential for patient and treatment response assessment, standardization of clinical and imaging follow-up protocols after transarterial locoregional therapy (LRT) of hepatic malignancies is hampered by a lack of supporting evidence in the IR literature to dictate suitable follow-up methods and limited guidance regarding posttreatment surveillance practices in current clinical practice guidelines . The development of standardized, evidence-based postprocedure surveillance practices after IO procedures is of paramount importance as these procedures have become integral to the care of the oncology patient. Although previous survey data have suggested variation in follow-up practices within the IR community , there remains a paucity of formally acquired information on the specific follow-up approaches used by IRs after IO therapies. This exploratory survey study thus aimed to characterize practices and quantify variation in follow-up approaches among IRs after liver transarterial LRTs in contemporary IO clinical practice to better understand currently used surveillance methods as a framework for future standardization of postprocedure care.
Materials and methods
Between November 24 and December 29, 2014, 3290 American and international Physician members of the Society of Interventional Radiology (SIR) (In-Training, Scientist, and Clinical Associate members excluded) were requested to participate in a survey ( Appendix ) designed by the SIR IO Service Line and disseminated through an online survey company (SurveyMonkey, Palo Alto, CA). Invitations were sent via electronic mail from the SIR on survey commencement and 3 weeks after survey outset as a reminder for participation. The order of survey questions was identical for all respondents. Two variant but structurally and substantively analogous parallel survey pathways were used, with the final five survey questions (21–25 vs. 26–30) differing for participants based on the individual response to question 20. Responses to the password-protected questionnaire were anonymous, and no compensation was provided for participation. Survey settings were structured to allow only one response per invited e-mail address to prevent duplicate responses. On survey closure, responses were compiled and analyzed.
Results
Get Radiology Tree app to read full this article<
Respondent Demographics
Get Radiology Tree app to read full this article<
Table 1
Demographics of Survey Respondents and Practice Settings
Member Characteristics SIR Members (n = 361) ∗ Years in practice <1 year 17 (5%) 1–5 years 92 (25%) 6–10 years 68 (19%) 11–15 years 56 (16%) >15 years 127 (35%) Practice location United States 311 (88%) Canada 8 (2%) Europe 14 (4%) South America 5 (1%) Asia 17 (5%) Practice type Community-based hospital 185 (54%) University-based (academic) hospital 153 (44%) Free-standing facility 8 (2%) Practice size Individual 17 (5%) 2 IRs 32 (9%) 3–5 IRs 182 (51%) 6–10 IRs 86 (24%) >10 IRs 41 (11%) Hospital size <200 beds 31 (9%) 201–400 beds 114 (32%) 401–600 beds 97 (27%) 601–800 beds 54 (15%) 801–1000 beds 35 (10%) >1000 beds 26 (7%) Liver transarterial therapies performed per month 1–5 196 (55%) 6–10 81 (23%) 11–15 31 (9%) >15 46 (13%)
SIR, Society of Interventional Radiology; IR, interventional radiologist.
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
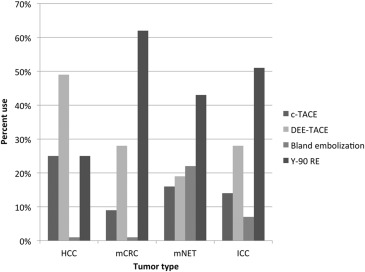
Transarterial LRT Procedures
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Clinical Follow-up
Frequency and Duration
Get Radiology Tree app to read full this article<
Laboratory Assessment
Get Radiology Tree app to read full this article<
Imaging Follow-up
Get Radiology Tree app to read full this article<
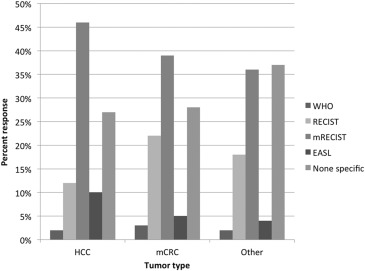
Follow-up Imaging Based on Tumor Type Treated
Get Radiology Tree app to read full this article<
Table 2
Preferred Imaging Follow-up Modality Based on Tumor Type Treated ∗ , †
Tumor Type CT MR Imaging PET-CT Ultrasound HCC 129/190 (68%) 144/190 (76%) 11/190 (6%) 1/190 (0.5%) mCRC 135/184 (73%) 71/184 (39%) 106/184 (58%) 2/184 (1%) mNET 140/183 (77%) 88/183 (48%) 34/183 (19%) 0/183 (0%) ICC 125/181 (69%) 119/181 (66%) 26/181 (14%) 0/181 (0%)
CT, computed tomography; MR, magnetic resonance; PET, positron emission tomography; HCC, hepatocellular carcinoma; mCRC, metastatic colorectal carcinoma; mNET, metastatic neuroendocrine tumor; ICC, intrahepatic cholangiocarcinoma.
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
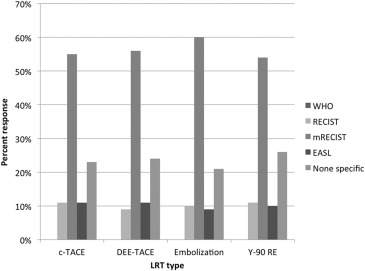
Follow-up Imaging Based on Transarterial LRT Used
Get Radiology Tree app to read full this article<
Table 3
Preferred Imaging Follow-up Modality Based on Transarterial LRT Used ∗ , †
Tumor Type CT MR Imaging PET-CT Ultrasound c-TACE 50/86 (58%) 44/86 (51%) 10/86 (12%) 2/86 (2%) DEB-TACE 48/83 (58%) 46/83 (55%) 12/83 (15%) 1/83 (1%) Bland embolization 46/79 (58%) 39/79 (49%) 8/79 (10%) 2/79 (3%) 90 Y RE 42/78 (54%) 38/78 (49%) 24/78 (31%) 0/78 (0%)
CT, computed tomography; MR, magnetic resonance; PET, positron emission tomography; c-TACE, conventional transarterial chemoembolization; DEB-TACE, drug-eluting bead transarterial chemoembolization; 90 Y RE, yttrium-90 radioembolization.
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Discussion
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Acknowledgments
Get Radiology Tree app to read full this article<
Supplementary data
Get Radiology Tree app to read full this article<
Appendix
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
References
1. Boas F.E., Do B., Louie J.D., et. al.: Optimal imaging surveillance schedules after liver-directed therapy for hepatocellular carcinoma. J Vasc Interv Radiol 2015; 26: pp. 69-73.
2. Bruix J., Sherman M., American Association for the Study of Liver D: Management of hepatocellular carcinoma: an update. Hepatology 2011; 53: pp. 1020-1022.
3. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2012; 56: pp. 908-943.
4. 2014. [cited 2015 August 13]; Available at: http://www.nccn.org/professionals/physician_gls/pdf/hepatobiliary.pdf
5. Gaba R.C.: Chemoembolization practice patterns and technical methods among interventional radiologists: results of an online survey. AJR Am J Roentgenol 2012; 198: pp. 692-699.
6. Brown D.B., Nikolic B., Covey A.M., et. al.: Quality improvement guidelines for transhepatic arterial chemoembolization, embolization, and chemotherapeutic infusion for hepatic malignancy. J Vasc Interv Radiol 2012; 23: pp. 287-294.
7. Ibrahim S.M., Nikolaidis P., Miller F.H., et. al.: Radiologic findings following Y90 radioembolization for primary liver malignancies. Abdom Imaging 2009; 34: pp. 566-581.
8. Salem R., Lewandowski R.J., Kulik L., et. al.: Radioembolization results in longer time-to-progression and reduced toxicity compared with chemoembolization in patients with hepatocellular carcinoma. Gastroenterology 2011; 140: pp. 497-507. e492
9. Zivin S.P., Bui J.T., Knuttinen M.G., et. al.: Timing of imaging response after locoregional transarterial therapy (LRT): comparison between transarterial chemoembolization (TACE), and yttrium-90 radioembolization (Y-90 RE). J Vasc Interv Radiol 2015; 26: pp. S60.
10. Shim J.H., Lee H.C., Kim S.O., et. al.: Which response criteria best help predict survival of patients with hepatocellular carcinoma following chemoembolization? A validation study of old and new models. Radiology 2012; 262: pp. 708-718.
11. Gillmore R., Stuart S., Kirkwood A., et. al.: EASL and mRECIST responses are independent prognostic factors for survival in hepatocellular cancer patients treated with transarterial embolization. J Hepatol 2011; 55: pp. 1309-1316.
12. Prajapati H.J., Spivey J.R., Hanish S.I., et. al.: mRECIST and EASL responses at early time point by contrast-enhanced dynamic MRI predict survival in patients with unresectable hepatocellular carcinoma (HCC) treated by doxorubicin drug-eluting beads transarterial chemoembolization (DEB TACE). Ann Oncol 2013; 24: pp. 965-973.
13. Salem R., Miller F.H., Yaghmai V., et. al.: Response assessment methodologies in hepatocellular carcinoma: complexities in the era of local and systemic treatments. J Hepatol 2013; 58: pp. 1260-1262.
14. Riaz A., Memon K., Miller F.H., et. al.: Role of the EASL, RECIST, and WHO response guidelines alone or in combination for hepatocellular carcinoma: radiologic-pathologic correlation. J Hepatol 2011; 54: pp. 695-704.