Rationale and Objectives
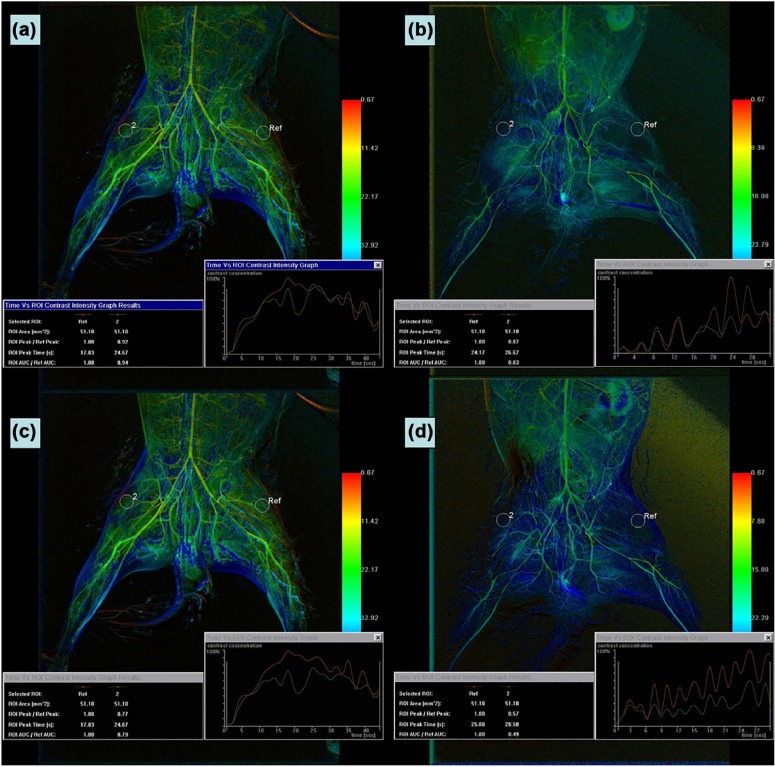
This paper describes an ongoing investigation of imaging and characterization of ischemia-reperfusion (IR) and investigated the use of color-coded digital subtraction angiography (DSA) to assess reperfusion injury or potential injury.
Methods
New Zealand white rabbits were subjected to right hindlimb ischemia (IR, n = 24) or sham operation (control, n = 6). After 3 hours, the IR rabbits underwent reperfusion and were assessed at 0, 6, 12, or 24 hours ( n = 6 each). DSA of the bilateral vastus lateralis muscle of each animal was performed. The maximum contrast enhancement value of a consistent region of interest in the right and left hind limbs (peak enhancement-R/L) was determined. Associations between the relative ratio of the peak right limb to the peak left limb (peak-R/L) and the following blood indicators of IR injury were analyzed: lactic dehydrogenase (LDH), creatine kinase (CK), malondialdehyde (MDA), and superoxide dismutase (SOD).
Results
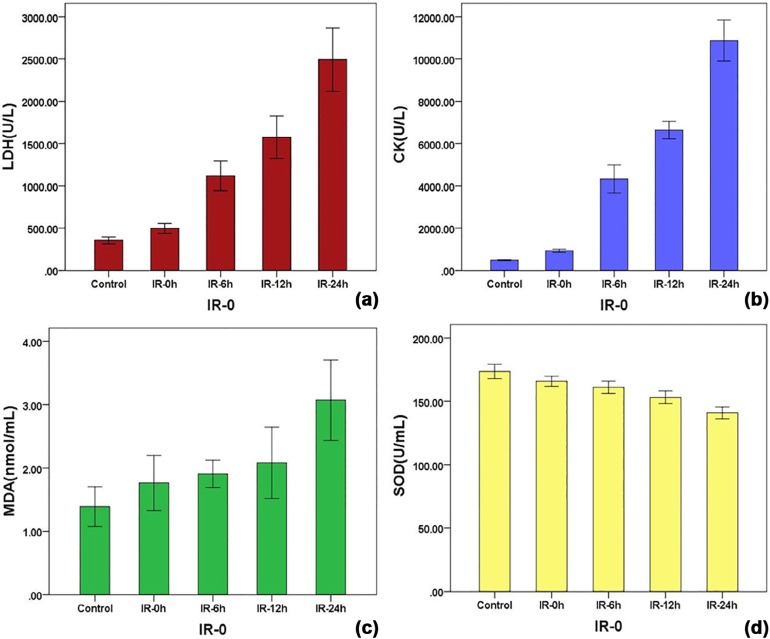
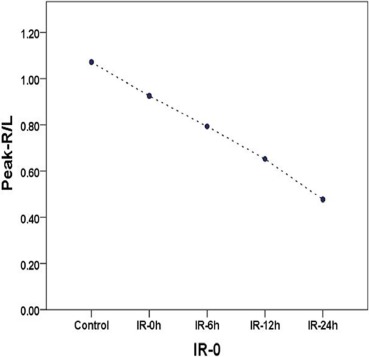
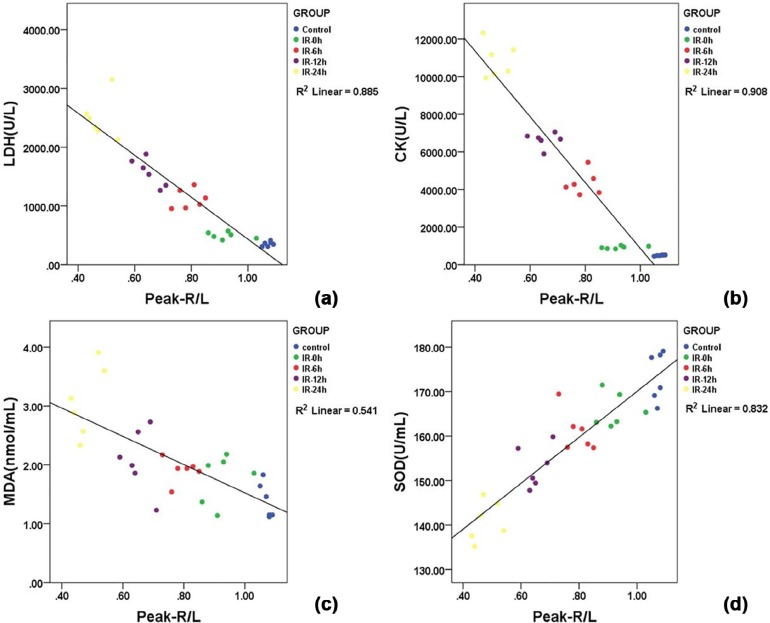
Serum LDH, CK, and MDA values in each IR group were significantly higher than those of the control group and were positively associated with the IR interval, whereas SOD was significantly lower and negatively associated. The mean peak-R/L decreased linearly with the IR interval from 1.07 ± 0.01 in the control group, and 0.93 ± 0.06, 0.79 ± 0.05, 0.65 ± 0.04, and 0.47 ± 0.04 at 0, 6, 12, and 24 hours in the IR groups. The coefficients of correlation between the peak-R/L and LDH, CK, MDA, SOD serum levels were −0.885, −0.908, −0.541, and 0.832, respectively.
Conclusions
Color-coded DSA may be used for monitoring the dynamics of skeletal muscle IR injury.
Introduction
Ischemia-reperfusion injury (IRI) occurs when there is a return of blood flow after a period of ischemia . IRI leads to cytokine activation and can result in multiple organ dysfunction . The local and systemic sequelae associated with reperfusion is only partially understood, although the technical success of recanalizing occluded arteries and re-establishing perfusion to ischemic limbs has improved significantly .
There are three methods for detecting IRI: clinical observation; biochemical laboratory abnormalities of malondialdehyde (MDA), superoxide dismutase (SOD), creatine kinase (CK), and lactic dehydrogenase (LDH) ; and imaging. Imaging modalities that enable visualization of the lesion, signs of tissue activity, perfusion, and metabolic status can be used to help detect IRI. These include computed tomography , magnetic resonance imaging , and radionuclide imaging , and are widely used for evaluating IRI of the myocardium and brain tissue . In addition to these modalities, digital subtraction angiography (DSA) provides an improved and instantaneous display of the characteristics and lesions of vessels . DSA is commonly used to perform angiography and to guide recanalization procedures in occluded arteries.
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Methods
Get Radiology Tree app to read full this article<
Experimental Groups
Get Radiology Tree app to read full this article<
Ischemia, Reperfusion, and Angiography
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Blood Indices
Get Radiology Tree app to read full this article<
Statistical Analysis
Get Radiology Tree app to read full this article<
Results
Animal Model of IR Verification
Get Radiology Tree app to read full this article<
Blood Indices and DSA Parameters
Get Radiology Tree app to read full this article<
TABLE 1
Serum Levels of LDH, CK, MDA, and SOD in Each Group
LDH (U/L) CK (U/L) MDA (nmol/mL) SOD (U/mL) Control 354.83 ± 40.08 535.65 ± 33.87 1.51 ± 0.24 173.54 ± 5.47 IR-0 495.83 ± 57.25 \* 929.17 ± 75.18 \* 1.99 ± 0.23 \* 165.79 ± 3.78 \* IR-6 1,117.33 ± 166.91 \* 4,330.22 ± 628.87 \* 2.11 ± 0.28 \* 161.06 ± 4.62 \* IR-12 1,574.33 ± 238.20 \* 6,636.88 ± 395.55 \* 2.42 ± 0.35 \* 153.14 ± 4.72 \* IR-24 2,492.33 ± 357.32 \* 10,875.13 ± 926.18 \* 3.07 ± 0.60 \* 140.88 ± 4.49 \*
CK, creatine kinase; IR, ischemia-reperfusion; IR-0, ischemia-reperfusion at 0 hour; IR-6, ischemia-reperfusion at 6 hours; IR-12, ischemia-reperfusion at 12 hours; IR-24, ischemia-reperfusion at 24 hours; LDH, lactic dehydrogenase; MDA, malondialdehyde; SOD, superoxide dismutase.
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Discussion
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Limitations
Get Radiology Tree app to read full this article<
Conclusions
Get Radiology Tree app to read full this article<
References
1. Falluji N., Mukherjee D.: Critical and acute limb ischemia: an overview. Angiology 2014; 65: pp. 137-146.
2. Yassin M.M., Harkin D.W., D’Sa A., et. al.: Lower limb ischemia-reperfusion injury triggers a systemic inflammatory response and multiple organ dysfunction. World J Surg 2002; 26: pp. 115-121.
3. McCord J.M.: Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med 1985; 312: pp. 159-163.
4. Hirsch A.T., Haskal Z.J., Hertzer N.R., et. al.: ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery. Circulation 2006; 113: pp. e463-e654.
5. Halladin N.L.: Oxidative and inflammatory biomarkers of ischemia and reperfusion injuries. Dan Med J 2015; 62: pp. B5054.
6. Anaya-Prado R., Toledo-Pereyra L.H., Lentsch A.B., et. al.: Ischemia/reperfusion injury. J Surg Res 2002; 105: pp. 248-258.
7. Brancaccio P., Maffulli N., Buonauro R., et. al.: Serum enzyme monitoring in sports medicine. Clin Sports Med 2008; 27: pp. 1-18.
8. Miles K.A., Hayball M., Dixon A.K.: Colour perfusion imaging: a new application of computed tomography. Lancet 1991; 337: pp. 643-645.
9. Loerakker S., Oomens C.W., Manders E., et. al.: Ischemia-reperfusion injury in rat skeletal muscle assessed with T2-weighted and dynamic contrast-enhanced MRI. Magn Reson Med 2011; 66: pp. 528-537.
10. Wiersema A.M., Oyen W., Dirksen R., et. al.: Early assessment of skeletal muscle damage after ischaemia-reperfusion injury using Tc-99m-glucarate. Cardiovasc Surg 2000; 8: pp. 186-191.
11. Koome M., Churilov L., Chen Z., et. al.: Computed tomography perfusion as a diagnostic tool for seizures after ischemic stroke. Neuroradiology 2016; 58: pp. 577-584.
12. Nakanishi R., Matsumoto S., Alani A., et. al.: Diagnostic performance of transluminal attenuation gradient and fractional flow reserve by coronary computed tomographic angiography (FFR(CT)) compared to invasive FFR: a sub-group analysis from the DISCOVER-FLOW and DeFACTO studies. Int J Cardiovasc Imaging 2015; 31: pp. 1251-1259.
13. Ahn S.S., Kim Y.D.: Three-dimensional digital subtraction angiographic evaluation of aneurysm remnants after clip placement. J Korean Neurosurg Soc 2010; 47: pp. 185-190.
14. Cheon J.E.: Quantitative digital subtraction angiography in pediatric moyamoya disease. J Korean Neurosurg Soc 2015; 57: pp. 432.
15. Strother C.M., Bender F., Deuerling-Zheng Y., et. al.: Parametric color coding of digital subtraction angiography. AJNR Am J Neuroradiol 2010; 31: pp. 919-924.
16. Kostrzewa M., Kara K., Pilz L., et. al.: Treatment evaluation of flow-limiting stenoses of the superficial femoral and popliteal artery by parametric color-coding analysis of digital subtraction angiography series. Cardiovasc Intervent Radiol 2017; 40: pp. 1147-1154.
17. Struffert T., Ott S., Kowarschik M., et. al.: Measurement of quantifiable parameters by time-density curves in the elastase-induced aneurysm model: first results in the comparison of a flow diverter and a conventional aneurysm stent. Eur Radiol 2013; 23: pp. 521-527.
18. Lin C., Luo C., Hung S., et. al.: Application of color-coded digital subtraction angiography in treatment of indirect carotid-cavernous fistulas: initial experience. J Chin Med Assoc 2013; 76: pp. 218-224.
19. Wang J., Cheng J.J., Huang K.Y., et. al.: Quantitative assessment of angiographic perfusion reduction using color-coded digital subtraction angiography during transarterial chemoembolization. Abdom Radiol (NY) 2016; 41: pp. 545-552.
20. Su H., Lou W., Gu J.: Clinical values of hemodynamics assessment by parametric color coding of digital subtraction angiography before and after endovascular therapy for critical limb ischaemia. Zhonghua Yi Xue Za Zhi 2015; 95: pp. 3036-3040.
21. Blaisdell F.W.: The pathophysiology of skeletal muscle ischemia and the reperfusion syndrome: a review. Cardiovasc Surg 2002; 10: pp. 620-630.
22. Belkin M., Brown R.D., Wright J.G., et. al.: A new quantitative spectrophotometric assay of ischemia-reperfusion injury in skeletal muscle. Am J Surg 1988; 156: pp. 83-86.
23. Chervu A., Moore W.S., Homsher E., et. al.: Differential recovery of skeletal muscle and peripheral nerve function after ischemia and reperfusion. J Surg Res 1989; 47: pp. 12-19.
24. Adeva M., Gonz lez-Lucn M., Seco M., et. al.: Enzymes involved in l-lactate metabolism in humans. Mitochondrion 2013; 13: pp. 615-629.
25. Saks V.A., Rosenshtraukh L., Smirnov V.N., et. al.: Role of creatine phosphokinase in cellular function and metabolism. Can J Physiol Pharmacol 1978; 56: pp. 691-706.
26. Eiken O., Nabseth D.C., Mayer R.F., et. al.: Limb replantation. II. The pathophysiological effects. Arch Surg 1964; 88: pp. 54-65.
27. Penneys R.: Serum lactic dehydrogenase (LDH) isozymes with ischemic damage of skeletal muscle of the human limb. Angiology 1967; 18: pp. 678-681.
28. Chiu D., Wang H.H., Blumenthal M.R.: Creatine phosphokinase release as a measure of tourniquet effect on skeletal muscle. Arch Surg 1976; 111: pp. 71-74.
29. Gawel S., Wardas M., Niedworok E., et. al.: Malondialdehyde (MDA) as a lipid peroxidation marker. Wiad Lek 2004; 57: pp. 453-455.
30. Wang X.T., Tian Y., Xu W.X., et. al.: Protective effects of modeled superoxide dismutase coordination compound (MSODa) against ischemia/reperfusion injury in rat skeletal muscle. Cell Physiol Biochem 2015; 37: pp. 465-476.
31. Widgerow A.D.: Ischemia-reperfusion injury: influencing the microcirculatory and cellular environment. Ann Plast Surg 2014; 72: pp. 253-260.