Rationale and Objectives
To compare free-breathing three-dimensional (3D) phase-sensitive inversion recovery (PSIR) with breath-holding two-dimensional (2D) IR sequences to determine which is better for detecting and characterizing myocardial late gadolinium enhancement (LGE) in hypertrophic cardiomyopathy (HCM) patients.
Materials and Methods
Thirty HCM patients clinically underwent 3.0 T cardiac magnetic resonance imaging that included 3D-PSIR and 2D-IR. The amount of LGE lesions was calculated and expressed as %LGE of the myocardial mass, and the average of the %LGE value reported by two observers was recorded as the final %LGE. We also counted the number of LGE lesions and recorded their location. The myocardium-LGE contrast, margin sharpness, artifacts, and overall image quality were graded on a 4-point grading scale (1 = poor, 2 = fair, 3 = good, 4 = excellent).
Results
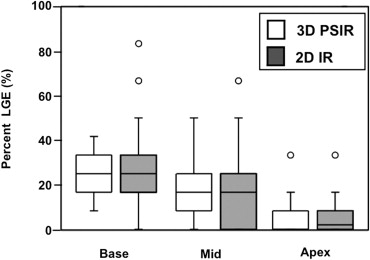
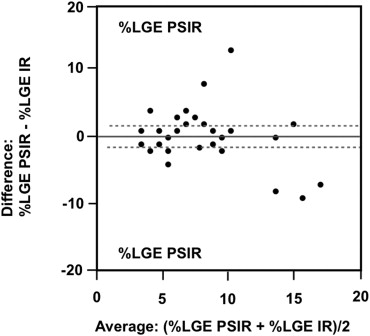
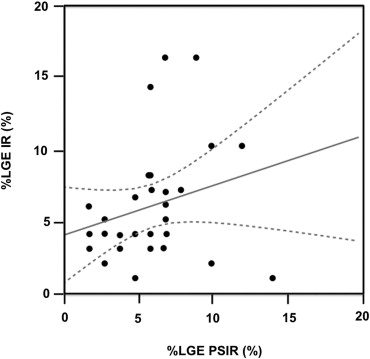
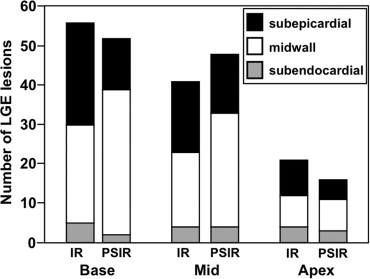
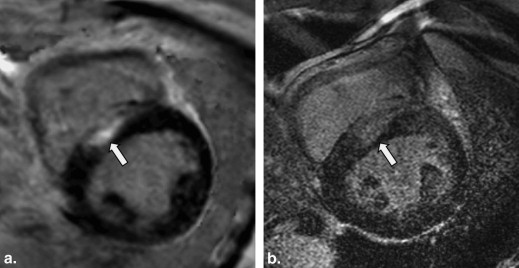
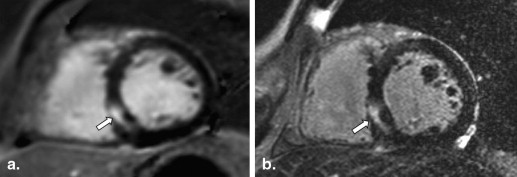
The mean %LGE on 2D-IR was 24.7 ± 0.6, 17.5 ± 0.6, and 8.5 ± 0.3, respectively, for the basal, mid-, and apical myocardium; the corresponding values were 24.2 ± 0.4, 20.0 ± 0.4, and 7.7 ± 0.3 on 3D-PSIR (2D-IR versus 3D-PSIR, P = .87). On 2D IR and 3D-PSIR images, 13, 52, and 53, and 9, 74, and 33 LGE lesions were detected in the subendocardial, midwall, subepicardial area, respectively. The myocardium-LGE contrast and overall image quality were significantly higher on 3D-PSIR than 2D-IR images ( P < .001); the sequences did not differ significantly with respect to margin sharpness and artifact.
Conclusion
Three-dimensional PSIR sequence yields higher image contrast, better image quality, and greater detection ability for LGE lesions than 2D-IR sequence.
Cardiovascular magnetic resonance (CMR) imaging at 1.5- and 3.0-T machines yields high spatial and contrast resolution and is now accepted as a valuable tool for the evaluation of many cardiac diseases . It is particularly useful for the assessment of cardiomyopathies because it can depict different myocardial enhancement patterns on inversion-recovery (IR) late gadolinium-enhanced (LGE) images . Patients with hypertrophic cardiomyopathy (HCM) have a genetically anomalous, usually hypercontractile and asymmetric, myocardium that may obstruct output and result in sudden cardiac death. The estimated incidence of HCM is 1 in 500 in the general population . Evidence has emerged that myocardial fibrosis is a relatively early manifestation of HCM and that it may have a pathophysiological role in sudden cardiac death . Adabag et al found that LGE was an independent risk factor for HCM that was reflected in a 7.3-fold increase in the RR interval during nonsustained ventricular tachycardia, a potential risk factor for sudden death . LGE CMR imaging can depict the peculiar pattern of fibrosis whose increase is observed in patients at risk for major cardiac events . Therefore, the evaluation of the distribution and extent of LGE is clinically important for risk stratification and the management of HCM patients.
At LGE-CMR studies, two-dimensional (2D) IR images are conventionally acquired under breath-holding and an optimal inversion recovery time (TI) must be selected. Myocardial fibrosis is located in the area of hypertrophy; it can be patchy with multiple foci or distributed diffusely . As small or patchy LGE lesions can be missed on conventional 2D IR images because of noncontiguous slice coverage attributable to misregistration between breath holds, three-dimensional (3D) phase-sensitive IR (PSIR) under free breathing has been proposed as a technique to detect LGE lesions . Advantages of this technique include the consistent contrast and appearance over a relatively wide range of TI . The introduction of 3.0-T MR machines has been increasing in the clinical settings; however, there were no reports that evaluated the potentials of PSIR sequence for diagnosis of cardiomyopathy at a 3.0-T machine.
Get Radiology Tree app to read full this article<
Materials and methods
Get Radiology Tree app to read full this article<
Patient Population
Get Radiology Tree app to read full this article<
MR Imaging
Get Radiology Tree app to read full this article<
Quantitative Evaluation
Get Radiology Tree app to read full this article<
Visual Evaluation
Get Radiology Tree app to read full this article<
Statistical Analysis
Get Radiology Tree app to read full this article<
Results
Quantitative Evaluation
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Visual Evaluation
Get Radiology Tree app to read full this article<
Table 1
Scores for the Visual Evaluation of LGE Lesions on 2D IR and 3D PSIR Images
2D IR 3D PSIR_P_ Value Contrast 2.3 ± 0.7 3.2 ± 0.5 <.001 Sharpness 2.4 ± 0.6 2.7 ± 0.6 .14 Artifact 2.2 ± 0.7 2.5 ± 0.7 .20 Overall 2.3 ± 0.6 2.8 ± 0.7 .006
2D, two-dimensional; 3D, three-dimensional; IR, inversion recovery; PSIR, phase-sensitive inversion recovery.
Get Radiology Tree app to read full this article<
Discussion
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
References
1. Mahrholdt H., Wagner A., Judd R.M., et. al.: Delayed enhancement cardiovascular magnetic resonance assessment of non-ischaemic cardiomyopathies. Eur Heart J 2005; 26: pp. 1461-1474.
2. Maron B.J., McKenna W.J., Danielson G.K., et. al.: American College of Cardiology/European Society of Cardiology Clinical Expert Consensus Document on Hypertrophic Cardiomyopathy. A report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents and the European Society of Cardiology Committee for Practice Guidelines. Eur Heart J 2003; 24: pp. 1965-1991.
3. Gersh B.J., Maron B.J., Bonow R.O., et. al.: 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2011; 124: pp. e783-e831.
4. Nael K., Fenchel M., Saleh R., et. al.: Cardiac MR imaging: new advances and role of 3T. Magn Reson Imaging Clin North Am 2007; 15: pp. 291-300. v
5. Araoz P.A., Glockner J.F., McGee K.P., et. al.: 3 Tesla MR imaging provides improved contrast in first-pass myocardial perfusion imaging over a range of gadolinium doses. J Cardiovasc Magn Reson 2005; 7: pp. 559-564.
6. Maron B.J., Gardin J.M., Flack J.M., et. al.: Prevalence of hypertrophic cardiomyopathy in a general population of young adults. Echocardiographic analysis of 4111 subjects in the CARDIA Study. Coronary Artery Risk Development in (Young) Adults. Circulation 1995; 92: pp. 785-789.
7. Ho C.Y., Lopez B., Coelho-Filho O.R., et. al.: Myocardial fibrosis as an early manifestation of hypertrophic cardiomyopathy. New Engl J Med 2010; 363: pp. 552-563.
8. Shirani J., Pick R., Roberts W.C., et. al.: Morphology and significance of the left ventricular collagen network in young patients with hypertrophic cardiomyopathy and sudden cardiac death. J Am Coll Cardiol 2000; 35: pp. 36-44.
9. Adabag A.S., Maron B.J., Appelbaum E., et. al.: Occurrence and frequency of arrhythmias in hypertrophic cardiomyopathy in relation to delayed enhancement on cardiovascular magnetic resonance. J Am Coll Cardiol 2008; 51: pp. 1369-1374.
10. Monserrat L., Elliott P.M., Gimeno J.R., et. al.: Non-sustained ventricular tachycardia in hypertrophic cardiomyopathy: an independent marker of sudden death risk in young patients. J Am Coll Cardiol 2003; 42: pp. 873-879.
11. Christiaans I., van Engelen K., van Langen I.M., et. al.: Risk stratification for sudden cardiac death in hypertrophic cardiomyopathy: systematic review of clinical risk markers. Europace 2010; 12: pp. 313-321.
12. Adabag A.S., Casey S.A., Kuskowski M.A., et. al.: Spectrum and prognostic significance of arrhythmias on ambulatory Holter electrocardiogram in hypertrophic cardiomyopathy. J Am Coll Cardiol 2005; 45: pp. 697-704.
13. Kim R.J., Judd R.M.: Gadolinium-enhanced magnetic resonance imaging in hypertrophic cardiomyopathy: in vivo imaging of the pathologic substrate for premature cardiac death?. J Am Coll Cardiol 2003; 41: pp. 1568-1572.
14. Bruder O., Wagner A., Jensen C.J., et. al.: Myocardial scar visualized by cardiovascular magnetic resonance imaging predicts major adverse events in patients with hypertrophic cardiomyopathy. J Am Coll Cardiol 2010; 56: pp. 875-887.
15. Kellman P., Larson A.C., Hsu L.Y., et. al.: Motion-corrected free-breathing delayed enhancement imaging of myocardial infarction. Magn Reson Med 2005; 53: pp. 194-200.
16. Kellman P., Arai A.E., McVeigh E.R., et. al.: Phase-sensitive inversion recovery for detecting myocardial infarction using gadolinium-delayed hyperenhancement. Magn Reson Med 2002; 47: pp. 372-383.
17. Cerqueira M.D., Weissman N.J., Dilsizian V., et. al.: Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart: a statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation 2002; 105: pp. 539-542.
18. Dietrich O., Raya J.G., Reeder S.B., et. al.: Measurement of signal-to-noise ratios in MR images: influence of multichannel coils, parallel imaging, and reconstruction filters. J Magn Reson Imaging 2007; 26: pp. 375-385.
19. Moon J.C., Reed E., Sheppard M.N., et. al.: The histologic basis of late gadolinium enhancement cardiovascular magnetic resonance in hypertrophic cardiomyopathy. J Am Coll Cardiol 2004; 43: pp. 2260-2264.
20. Greenbaum R.A., Ho S.Y., Gibson D.G., et. al.: Left ventricular fibre architecture in man. Br Heart J 1981; 45: pp. 248-263.
21. Kuribayashi T., Roberts W.C.: Myocardial disarray at junction of ventricular septum and left and right ventricular free walls in hypertrophic cardiomyopathy. Am J Cardiol 1992; 70: pp. 1333-1340.
22. Kino A., Keeling A.N., Farrelly C.T., et. al.: Assessment of left ventricular myocardial scar in infiltrative and non-ischemic cardiac diseases by free breathing three dimensional phase sensitive inversion recovery (PSIR) TurboFLASH. Int J Cardiovasc Imaging 2011; 27: pp. 527-537.
23. Peukert D., Laule M., Taupitz M., et. al.: 3D and 2D delayed-enhancement magnetic resonance imaging for detection of myocardial infarction: preclinical and clinical results. Acad Radiol 2007; 14: pp. 788-794.
24. Ozgun M., Maintz D., Bunck A.C., et. al.: Right atrial scar detection after catheter ablation: comparison of 2D and high spatial resolution 3D-late enhancement magnetic resonance imaging. Acad Radiol 2011; 18: pp. 488-494.
25. Sorajja P., Chareonthaitawee P., Ommen S.R., et. al.: Prognostic utility of single-photon emission computed tomography in adult patients with hypertrophic cardiomyopathy. Am Heart J 2006; 151: pp. 426-435.
26. Kaimoto S., Kawasaki T., Kuribayashi T., et. al.: Myocardial perfusion abnormality in the area of ventricular septum-free wall junction and cardiovascular events in nonobstructive hypertrophic cardiomyopathy. Int J Cardiovasc Imaging 2011; 28: pp. 1829-1839.
27. Hashimoto J., Ogawa K., Kubo A., et. al.: Application of transmission scan-based attenuation compensation to scatter-corrected thallium-201 myocardial single-photon emission tomographic images. Eur J Nucl Med 1998; 25: pp. 120-127.
28. Prvulovich E.M., Lonn A.H., Bomanji J.B., et. al.: Effect of attenuation correction on myocardial thallium-201 distribution in patients with a low likelihood of coronary artery disease. Eur J Nucl Med 1997; 24: pp. 266-275.