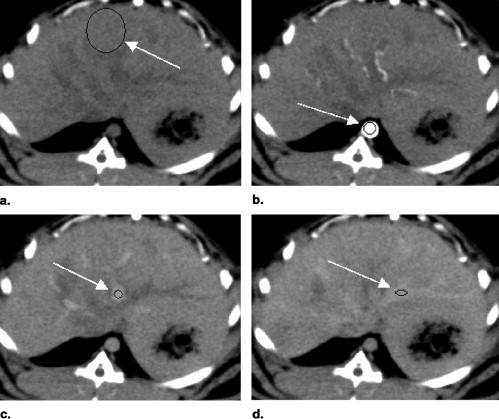
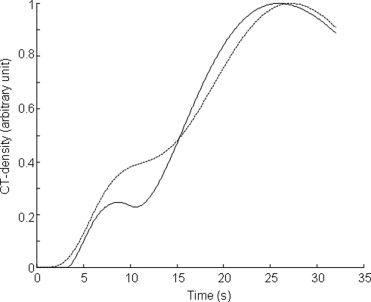
Rationale and Objectives
To compare liver perfusion parameters obtained by using an extravascular contrast agent and a blood-pool agent.
Materials and Methods
Fifteen rabbits were imaged with a continuous 40-second single-slice computed tomography acquisition after a bolus injection of contrast agent (physiologic bolus duration 4–5 seconds, extravascular iohexol, n = 7; experimental nanoparticulated blood-pool agent WIN8883, n = 8). Time-density curves were generated for the aorta, portal vein, and liver. From the curves, arterial, portal, and total blood flows and hepatic perfusion index (HPI, arterial-to-total perfusion ratio) were determined by using two commonly applied fundamentally different analyzing methods: the single-compartment model and the peak gradient (PG) method. Also, the gamma variate fitting method was used.
Results
By using the single-compartment model, the obtained HPI and total blood flow were 0.14 ± 0.04 and 2.29 ± 0.40 (mL/min/mL tissue ) for WIN8883, and 0.15 ± 0.06 ( P = .54) and 4.60 ± 1.14 (mL/min/mL tissue ) ( P = .0002) for iohexol, respectively. With the PG, HPI and total blood flow were 0.15 ± 0.08 and 1.27 ± 0.24 (mL/min/mL tissue ) for WIN8883, and 0.20 ± 0.06 ( P = .12) and 2.11 ± 0.25 (mL/min/mL tissue ) ( P = .00002) for iohexol, respectively. With the blood pool agent, similar contrast enhancement to the conventional agent was achieved with about 36% reduced dosage of iodine per body weight (mg I/kg).
Conclusions
HPI was found to be quite insensitive to different contrast agent types and analyzing methods. However, the arterial, portal and total liver blood flow values strongly depend on contrast agent type and modeling method.
The determination of liver perfusion has received increasing attention because most hepatic diseases cause alterations in perfusion conditions ( ). With liver metastases, these alterations seem to occur even before any structural changes become visible ( ). Perfusion computed tomography (CT) offers a clinical tool for the determination of both global and local perfusion changes within the liver with high spatial and temporal resolutions ( ). Measuring blood volume and flow with functional computed tomography (fCT) uses a modality specific marker introduced into the bloodstream coupled with mathematical modeling of the behavior of the marker as it circulates through the organ under investigation ( ). Validity to report physiologically meaningful perfusion parameters and the comparability of these parameters obtained with different imaging and analyzing procedures are important challenges in hepatic perfusion imaging ( ).
Clinically used iodinated CT contrast agents readily pass across the capillary membranes of most organs ( ). Contrast agents with their distribution limited to vascular space have been under development for CT ( ). With such agents, vascular-specific contrast is obtained in addition to long-lasting tissue enhancement. They also enable the use of simple and clinically attractive perfusion analyzing methodology. One of the most important questions for clinical practise is the relation of perfusion parameters obtained with conventional extravascular agents and intravascular contrast agents. Such comparable knowledge of these parameters with both agent types is potentially helpful in future studies conducted with either agent.
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Material and methods
Study Population and Contrast Material
Get Radiology Tree app to read full this article<
CT Scanning
Get Radiology Tree app to read full this article<
Data Analysis and Image Evaluation
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Results
Get Radiology Tree app to read full this article<
Table 1
Perfusion Parameters Obtained With Iohexol and WIN8883 by Using the Single-Compartment Model
F arterial F portal F total Hepatic Perfusion Index Mean Transit Time WIN8883 0.34 ± 0.13 1.95 ± 0.36 2.29 ± 0.40 0.14 ± 0.04 8.2 ± 1.8 Iohexol 0.67 ± 0.25 3.93 ± 1.08 4.60 ± 1.14 0.15 ± 0.06 7.0 ± 2.8P .007 .0005 .0002 .54 .85
The blood flows are given in mL/min/mL tissue and mean transit time in seconds.
Table 2
Perfusion Parameters Obtained With Iohexol and WIN8883 by Using the Peak Gradient (PG) Method
F arterial F portal F total Hepatic Perfusion Index WIN8883 0.20 ± 0.13 1.08 ± 0.20 1.27 ± 0.24 0.15 ± 0.08 Iohexol 0.43 ± 0.13 1.69 ± 0.25 2.11 ± 0.25 0.20 ± 0.06P .005 .0002 .00002 .12
The blood flows are given in mL/min/mL tissue .
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Table 3
Normalized Steepness (the Time Gradient Divided by the Peak Enhancement) of Aortic, Portal Vein, Hepatic Arterial, and Portal Phase Enhancement Curves
Aorta Portal Vein Arterial Phase Portal Phase WIN8883 0.72 ± 0.06 0.28 ± 0.06 0.29 ± 0.05 0.13 ± 0.04 Iohexol 0.66 ± 0.06 0.16 ± 0.03 0.32 ± 0.08 0.084 ± 0.008P .21 .006 .54 .03
The values are in s – 1 .
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Table 4
The Maximal ΔHU Values of the Enhancement Curves Measured From Aorta, Portal Vein, and Hepatic Arterial and Portal Phases
Aorta Portal Vein Arterial Phase Portal Phase WIN8883 1241 ± 657 272 ± 132 9.9 ± 3.1 38.5 ± 11.6 Iohexol 356 ± 44 67 ± 18 8.2 ± 3.4 24.6 ± 3.9P .007 .003 .37 .017
Get Radiology Tree app to read full this article<
Discussion
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Appendix 1
Get Radiology Tree app to read full this article<
VdC(t)dt=FACA(t)+FPCP(t)−VMTTC(t) V
d
C
(
t
)
d
t
=
F
A
C
A
(
t
)
+
F
P
C
P
(
t
)
−
V
MTT
C
(
t
)
V is the volume [mL tissue ] of the volume of interest (VOI); MTT is the mean transit time for the contrast agent to move through the VOI [seconds]; F A , F P , and F (= F A + F P ) [mL/second] are hepatic arterial, portal, and total blood flows; and C A ( t ), C P ( t ), and C ( t ) are the contrast material concentrations in the aorta, portal, vein, and the VOI, respectively. Morales and Smith ( ) have suggested that a contrast agent gradient between tissue input and output causes that a true blood flow is related to the obtained blood flow as F obtained = F true / r , with 0 ≤ r ≤ 1, resulting in that F obtained ≥ F true .
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
F′V=dHtissue(t)dtHinput(t) F
′
V
=
d
H
t
i
s
s
u
e
(
t
)
d
t
H
i
n
p
u
t
(
t
)
where F ′ is arterial or portal blood flow [mL/second], H tissue is arterial or portal bolus phase ΔHU time data, and H input is ΔHU time data of arterial or portal phase bolus input measured from aorta or portal vein, respectively.
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
F′V=Htissue(t)∫0tHinput(t)dt−∫0tHoutput(t)dt F
′
V
=
H
t
i
s
s
u
e
(
t
)
∫
0
t
H
i
n
p
u
t
(
t
)
d
t
−
∫
0
t
H
o
u
t
p
u
t
(
t
)
d
t
where integration is carried out from the beginning of the upslope of the bolus to the time t . For arterial blood flow, we integrated from zero to the maximum of arterial phase assuming no outflow. For portal blood flow, we integrated from zero to the 50% value of portal phase assuming no outflow. We also used outflow corrected version of this equation for WIN8883 by taking H output from the ΔHU time data measured from hepatic vein.
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
MTT=VF MTT
=
V
F
Get Radiology Tree app to read full this article<
References
1. Pandharipande P.V., Krinsky G.A., Rusinek H., et. al.: Perfusion imaging of the liver: current challenges and future goals. Radiology 2005; 234: pp. 661-673.
2. Miles K.A., Griffiths M.R.: Perfusion CT: a worthwhile enhancement?. Br J Radiol 2003; 76: pp. 220-231.
3. Platt J.F., Francis I.R., Ellis J.H., et. al.: Liver metastases: early detection based on abnormal contrast material enhancement at dual-phase helical CT. Radiology 1997; 205: pp. 49-53.
4. Leen E.: The detection of occult liver metastases of colorectal carcinoma. J Hepatobil Pancreat Surg 1999; 1: pp. 7-15.
5. Lee T.Y.: Functional CT: physiological models. Trends Biotechnol 2002; 20: pp. S3-S10.
6. Canty J.M., Judd R.M., Brody A.S., et. al.: First pass entry of non-ionic contrast agent into the myocardial extravascular space. Circulation 1991; 84: pp. 2071-2078.
7. Idee J., Port M., Robert P., et. al.: Preclinical profile of the monodisperse iodinated macromolecular blood pool agent P743. Invest Radiol 2001; 36: pp. 41-49.
8. Gazelle G.S., Wolf G.L., McIntire G.L., et. al.: Nanoparticulate computed tomography contrast agents for blood pool and liver-spleen imaging. Acad Radiol 1994; 1: pp. 373-376.
9. Materne R., Van Beers B.E., Smith A.M., et. al.: Non-invasive quantification of liver perfusion with dynamic computed tomography and a dual-input one-compartmental model. Clin Sci 2000; 99: pp. 517-525.
10. Van Beers B., Leconte I., Materne R., et. al.: Hepatic perfusion parameters in chronic liver disease: dynamic CT measurements correlated with disease severity. Am J Roentgenol 2001; 176: pp. 667-673.
11. Miles K.A., Hayball M.P., Dixon A.K.: Functional images of hepatic perfusion obtained with dynamic CT. Radiol 1993; 188: pp. 405-411.
12. Miles K.A.: Measurement of tissue perfusion by dynamic computed topography. Br J Radiol 1991; 64: pp. 409-412.
13. Bader T.R., Herneth A.M., Blaicher W., et. al.: Hepatic perfusion after liver transplantation: noninvasive measurement with dynamic single-section CT. Radiology 1998; 209: pp. 129-134.
14. Blomley M.J.K., Coulden R., Dawson P., et. al.: Liver perfusion studied with ultrafast CT. J Comp Assist Tomogr 1995; 19: pp. 424-433.
15. Wolf G.L., Gazelle G.S., McIntire G.L., et. al.: Percutaneous computed tomography lymphography in the rabbit by subcutaneously injected nanoparticles. Acad Radiol 1994; 1: pp. 352-357.
16. Mullani N., Gould K.L.: First pass measurements of regional blood flow using external detectors. J Nucl Med 1983; 24: pp. 577-581.
17. Halavaara J.T., Hamberg L.H., Leong F., et. al.: Functional CT in the assessment of liver vascular physiology. Acad Radiol 1996; 3: pp. 946-952.
18. Kapanen M.K., Halavaara J.T., Häkkinen A.M.: Open four-compartment model in the measurement of liver perfusion. Acad Radiol 2005; 12: pp. 1542-1550.
19. Kapanen M.K., Halavaara J.T., Häkkinen A.M.: Assessment of vascular physiology of tumorous livers: comparison of two different methods. Acad Radiol 2003; 10: pp. 1021-1029.
20. Goresky C.A.: A linear method for determining liver sinusoidal and extravascular volumes. Am J Physiol 1963; 204: pp. 626-640.
21. Bell S.D., Peters A.M.: Measurement of blood flow from first-pass radionuclide angiography: influence of bolus volume. Eur J Nucl Med 1991; 18: pp. 885-888.
22. Cuenod C.A., Leconte I., Siauve N., et. al.: Early changes in liver perfusion caused by occult metastases in rats: detection with quantitative CT. Radiology 2001; 218: pp. 556-561.
23. Cuenod C.A., Leconte I., Siauve N., et. al.: Deconvolution technique for measuring tissue perfusion by dynamic CT: application to normal and metastatic liver. Acad Radiol 2002; 9: pp. S205-S211.
24. Fournier L.S., Cuenod C.A., de Bazelaire C., et. al.: Early modifications of hepatic perfusion measured by functional CT in a rat model of hepatocellular carcinoma using a blood pool contrast agent. Eur Radiol 2004; 14: pp. 2125-2133.
25. Materne R., Smith A.M., Peeters F., et. al.: Assessment of hepatic perfusion parameters with dynamic MRI. Magn Reson Med 2002; 47: pp. 135-142.
26. Morales M.F., Smith R.E.: On the theory of blood-tissue exchange of inert gases. VI. Validity of approximate uptake expressions. Bull Math Biophys 1948; 10: pp. 191-200.
27. Meier P., Zierler K.: On the theory of the indicator dilution method for measurement of blood flow and volume. J Appl Physiol 1954; 6: pp. 731-744.