Rationale and Objectives
To prospectively investigate and compare three techniques of region of interest (ROI) placement for apparent diffusion coefficient (ADC) measurements in patients with pancreatic ductal adenocarcinoma (PDAC).
Materials and Methods
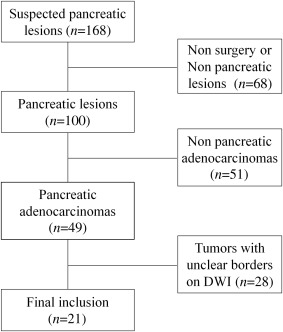
Twenty-one patients with surgical pathology–proven PDAC and 18 healthy volunteers were included. Respiratory-triggered single-shot echo-planar diffusion-weighted imaging ( b values = 0, 600 s/mm 2 ) was used to calculate the ADC maps across all participants. Three readers independently measured the ADCs according to three ROI methods: whole-volume, single-slice, and small solid samples of tumor. Mean ADCs for the healthy pancreas were calculated using three measurements from pancreatic head to tail, and ADCs of distal pancreas to the tumor were also measured. The interobserver variability for the three techniques was measured using the interclass correlation coefficient. The diagnostic performances were calculated and compared using the receiver operating characteristic curves (ROC).
Results
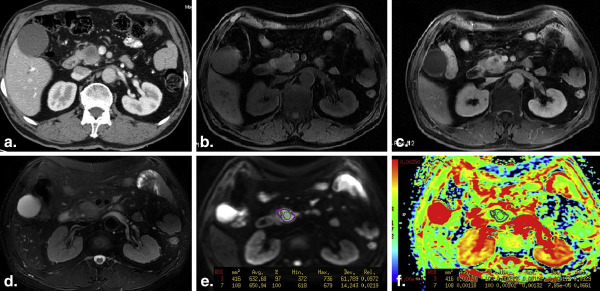
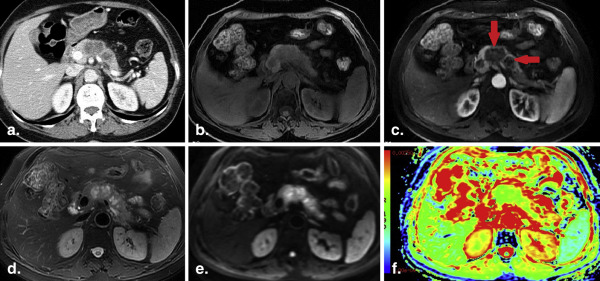
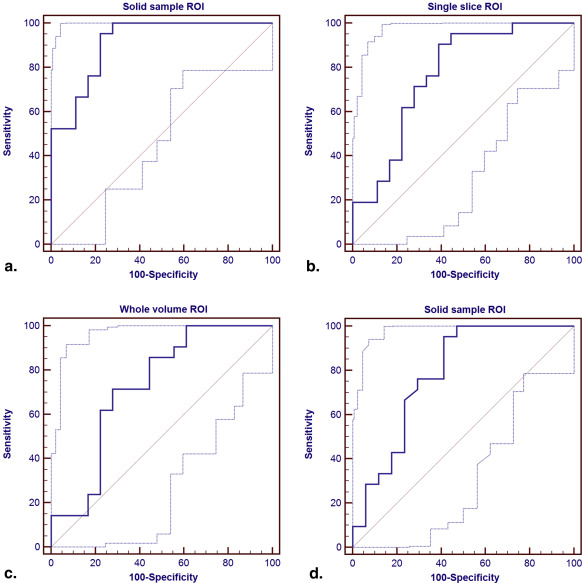
All the ADCs measured from the three ROI placements on PDAC were significantly lower than that from the normal pancreas. ADCs of solid tumor samples were significantly lower than that measured from whole volume or single slice (both P < .001). Only the ADCs measured from the solid sample ROI placements on tumor were observed significantly lower than the ADC of distal pancreatic parenchyma ( P = .005). Areas under the ROC for the identification of PDAC, based on small solid samples, single-slice and whole-volume ROIs, respectively, were 0.939, 0.791, and 0.735.
Conclusions
ADC based on the small solid samples of tumor provided the highest diagnostic performance in assessing PDAC and was more accurate than ADCs measured from single-slice or whole-volume ROI.
Pancreatic cancer accounts for about 3% of all cancer cases . It is one of the few cancers which have shown little improvement in survival rate (<6% of 5-year survival rate) over the past 40 years . About 74% patients with pancreatic cancer died within the first year of diagnosis . Magnetic resonance imaging (MRI) already plays an important role in detecting and differentiating pancreatic diseases, the recent emergence of diffusion-weighted imaging (DWI) provides an additional promising dimension to the conventional anatomic MRI. In particular, several studies have indicated that DWI could be promising in imaging pancreatic diseases. For instance, significantly lower ADC in pancreatic cancer than in benign pancreas tissue has been reported .
In addition to reflecting the physical properties of tissues, ADC values can be influenced by the techniques of region of interest (ROI) placement. For tumors, three techniques including the whole-volume , only a single-slice , and small sample ROIs of tumor have been used for the ADC measurements. However, whether ROIs for ADC measurements should ideally incorporate the entire tumor volume or only a representative tumor section of pancreatic cancer is still unclear. In addition, to our knowledge, no previous study has addressed the impact of the three ROI placement approaches for pancreatic tumor ADC measurements and diagnostic performances of the three ADC measurements.
Get Radiology Tree app to read full this article<
Materials and methods
Subjects
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Image Acquisition
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Table 1
The Main Parameters of Magnetic Resonance Sequences
Sequence TR/TE (ms) FOV (mm) Matrix Thickness/Gap (mm) Flip Angle ( 0 ) Slices NEX Band Width (KHz) Speed Factor 2D single-shot fast spin echo (MRCP) 7000/1253.4 300 × 300 288 × 288 64/0 — 6 0.92 31.2 — Axial fast spin echo (T2WI) 6316/73.8 360–400 320 × 192 5/1 90 20 2 83.3 2 Axial single-shot echo planar imaging (DWI) 6000/58.6 380 × 304 128 × 96 5/1 90 20 2/4 ∗ 250 2 3D fat-suppressed gradient-echo (LAVA) 2.5/1.1 440 × 418 256 × 180 2.5/0 11 84 0.70 125 2
DWI, diffusion-weighted imaging; FOV, field of view; LAVA, liver acceleration volume acquisition; MRCP, magnetic resonance cholangiopancreatography; TE, echo time; TR, repetition time.
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Image Analysis
Qualitative Analysis
Get Radiology Tree app to read full this article<
Quantitative Analysis
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Statistical Analysis
Get Radiology Tree app to read full this article<
Results
Get Radiology Tree app to read full this article<
Table 2
Comparisons of ADCs Measured From the Three ROI Methods Including the Whole Volume, a Single Slice, and Small Samples of Pancreatic Adenocarcinoma and Healthy Pancreas
Parameter Pancreatic Cancer; (Mean ± SD) Healthy Pancreas; (Mean ± SD)P Whole-volume ROIs ADCs (×10 −3 mm 2 /s) 1.43 ± 0.10 1.56 ± 0.19.022 Total ROI size (mm 2 ) 1978 ± 1217 103 ± 36<.001 Single-slice ROIs ADCs (×10 −3 mm 2 /s) 1.38 ± 0.14 1.56 ± 0.19.006 Total ROI size (mm 2 ) 588 ± 267 103 ± 36<.001 Solid sample ROIs ADCs (×10 −3 mm 2 /s) 1.27 ± 0.12 ∗ † 1.56 ± 0.19<.001 Total ROI size (mm 2 ) 58 ± 28 103 ± 36<.001
ADC, apparent diffusion coefficient; ROI, region of interest; SD, standard deviation.
Bold indicates statistically significant difference of P value <.05.
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Table 3
Results From the ROC Analyses of the Three ROI Methods Derived ADCs to Distinguish Between Pancreatic Adenocarcinoma and Healthy Pancreas
ROI Method Optimal Cutoff Values (×10 −3 mm 2 /s) AUC ± SE (95% CI) Specificities (95% CI) Sensitivities (95% CI) PPV (%) NPV (%) ACC (%) Whole-volume ROIs 1.48 0.725 ± 0.088 (0.559–0.855) 72.2 (46.5–90.3) 71.4 (47.8–88.7) 75.0 68.4 71.8 Single-slice ROIs 1.49 0.767 ± 0.081 (0.604–0.887) 61.1 (35.7–82.7) 90.5 (69.6–98.8) 73.1 84.6 76.9 Solid sample ROIs 1.42 0.913 ± 0.046 ∗ (0.778–0.979) 77.8 (52.4–93.6) 95.2 (76.2–99.9) 83.3 93.3 87.2 Solid sample ROIs † 1.42 0.783 ± 0.081 (0.619–0.900) 58.8 (32.9–81.6) 95.2 (76.2–99.9) 74.3 92.6 79.5
ACC, accuracy; ADC, apparent diffusion coefficient; AUC, area under curve; CI, confidence interval; NPV, negative predictive value; PPV, positive predictive value; ROC, operating characteristic curve; ROI, region of interest; SE, standard error.
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Discussion
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
References
1. International agency for research on cancer, WHO. Available at: http://eco.iarc.fr/eucan/Cancer.aspx?Cancer .
2. Siegel R., Naishadham D., Jemal A.: Cancer statistics, 2013. CA Cancer J Clin 2013; 63: pp. 11-30.
3. Matsuki M., Inada Y., Nakai G., et. al.: Diffusion-weighed MR imaging of pancreatic carcinoma. Abdom Imaging 2007; 32: pp. 481-483.
4. Ichikawa T., Erturk S.M., Motosugi U., et. al.: High b-value diffusion-weighted MRI for detecting pancreatic adenocarcinoma: preliminary results. AJR Am J Roentgenol 2007; 188: pp. 409-414.
5. Wiggermann P., Grützmann R., Weissenböck A., et. al.: Apparent diffusion coefficient measurements of the pancreas, pancreas carcinoma, and mass-forming focal pancreatitis. Acta Radiol 2012; 53: pp. 135-139.
6. Wang Y., Miller F.H., Chen Z.E., et. al.: Diffusion-weighted MR imaging of solid and cystic lesions of the pancreas. Radiographics 2011; 31: pp. E47-E64.
7. Lee S.S., Byun J.H., Park B.J., et. al.: Quantitative analysis of diffusion-weighted magnetic resonance imaging of the pancreas: usefulness in characterizing solid pancreatic masses. J Magn Reson Imaging 2008; 28: pp. 928-936.
8. Lemke A., Laun F.B., Klauss M., et. al.: Differentiation of pancreas carcinoma from healthy pancreatic tissue using multiple b-values: comparison of apparent diffusion coefficient and intravoxel incoherent motion derived parameters. Invest Radiol 2009; 44: pp. 769-775.
9. Rosenkrantz A.B., Matza B.W., Sabach A., et. al.: Pancreatic cancer: Lack of association between apparent diffusion coefficient values and adverse pathological features. Clin Radiol 2013; 68: pp. e191-e197.
10. Kamisawa T., Takuma K., Anjiki H., et. al.: Differentiation of autoimmune pancreatitis from pancreatic cancer by diffusion-weighted MRI. Am J Gastroenterol 2010; 105: pp. 1870-1875.
11. Kartalis N., Lindholm T.L., Aspelin P., et. al.: Diffusion-weighted magnetic resonance imaging of pancreas tumours. Eur Radiol 2009; 19: pp. 1981-1990.
12. Muraoka N., Uematsu H., Kimura H., et. al.: Apparent diffusion coefficient in pancreatic cancer: characterization and histopathological correlations. J Magn Reson Imaging 2008; 27: pp. 1302-1308.
13. Fattahi R., Balci N.C., Perman W.H., et. al.: Pancreatic diffusion-weighted imaging (DWI): comparison between mass-forming focal pancreatitis (FP), pancreatic cancer (PC), and normal pancreas. J Magn Reson Imaging 2009; 29: pp. 350-356.
14. Fukukura Y., Takumi K., Kamimura K., et. al.: Pancreatic adenocarcinoma: variability of diffusion-weighted MR imaging findings. Radiology 2012; 263: pp. 732-740.
15. Kang K.M., Lee J.M., Yoon J.H., et. al.: Intravoxel incoherent motion diffusion-weighted MR imaging for characterization of focal pancreatic lesions. Radiology 2014; 270: pp. 444-453.
16. Koc Z., Erbay G.: Optimal b value in diffusion-weighted imaging for differentiation of abdominal lesions. J Magn Reson Imaging 2014; 40: pp. 559-566.
17. Concia M., Sprinkart A.M., Penner A.H., et. al.: Diffusion-weighted magnetic resonance imaging of the pancreas: diagnostic benefit from an intravoxel incoherent motion model-based 3 b-value analysis. Invest Radiol 2014; 49: pp. 93-100.
18. Sun Y.S., Zhang X.P., Tang L., et. al.: Locally advanced rectal carcinoma treated with preoperative chemotherapy and radiation therapy: preliminary analysis of diffusion-weighted MR imaging for early detection of tumor histopathologic downstaging. Radiology 2010; 254: pp. 170-178.
19. Kim S.H., Lee J.Y., Lee J.M., et. al.: Apparent diffusion coefficient for evaluating tumour response to neoadjuvant chemoradiation therapy for locally advanced rectal cancer. Eur Radiol 2011; 21: pp. 987-995.
20. Cohen J.: Weighted kappa: nominal scale agreement with provision for scaled disagreement or partial credit. Psychol Bull 1968; 70: pp. 213-220.
21. Willemink M.J., Borstlap J., Takx R.A., et. al.: The effects of computed tomography with iterative reconstruction on solid pulmonary nodule volume quantification. PLoS One 2013; 8: pp. e58053.
22. Dale B.M., Braithwaite A.C., Boll D.T., et. al.: Field strength and diffusion encoding technique affect the apparent diffusion coefficient measurements in diffusion-weighted imaging of the abdomen. Invest Radiol 2010; 45: pp. 104-108.
23. Braithwaite A.C., Dale B.M., Boll D.T., et. al.: Short- and midterm reproducibility of apparent diffusion coefficient measurements at 3.0-T diffusion-weighted imaging of the abdomen. Radiology 2009; 250: pp. 459-465.
24. Kartalis N., Loizou L., Edsborg N., et. al.: Optimising diffusion-weighted MR imaging for demonstrating pancreatic cancer: a comparison of respiratory-triggered, free-breathing and breath-hold techniques. Eur Radiol 2012; 22: pp. 2186-2192.
25. Ma C., Pan C.S., Zhang H.G., et. al.: Diffusion-weighted MRI of the normal adult pancreas: the effect of age on apparent diffusion coefficient values. Clin Radiol 2013; 68: pp. e532-e537.
26. Wang Y., Chen Z.E., Nikolaidis P., et. al.: Diffusion-weighted magnetic resonance imaging of pancreatic adenocarcinomas: association with histopathology and tumor grade. J Magn Reson Imaging 2011; 33: pp. 136-142.
27. Momtahen A.J., Balci N.C., Alkaade S., et. al.: Focal pancreatitis mimicking pancreatic mass: magnetic resonance imaging (MRI)/magnetic resonance cholangiopancreatography (MRCP) findings including diffusion-weighted MRI. Acta Radiol 2008; 49: pp. 490-497.
28. Zhang J.L., Sigmund E.E., Rusinek H., et. al.: Optimization of b-value sampling for diffusion-weighted imaging of the kidney. Magn Reson Med 2012; 67: pp. 89-97.
29. Legrand L., Duchatelle V., Molinié V., et. al.: Pancreatic adenocarcinoma: MRI conspicuity and pathologic correlations. Abdom Imaging 2015; 40: pp. 85-94.
30. Barral M., Taouli B., Guiu B., et. al.: Diffusion-weighted MR imaging of the pancreas: current status and recommendations. Radiology 2015; 274: pp. 45-63.