Rationale and Objectives
Magnetic resonance (MR) perfusion techniques and MR spectroscopy (MRS) provide specific physiological information that may allow distinction between recurrent glioma and progression from stable disease.
Materials and Methods
Forty patients underwent conventional MR imaging, dynamic contrast-enhanced T1-weighted perfusion imaging, dynamic susceptibility contrast-enhanced perfusion imaging (DSC), and multivoxel MRS. Arterial spin labeling was available in 26 of these patients. Quantitative parameters were calculated in tumor recurrences and stable disease, which were retrospectively verified on clinical and radiological follow-up. Receiver operating characteristic curves for each parameter were generated for the differentiation between recurrent glioma and stable disease. A forward discriminant analysis was undertaken to assess the power of the conjunction of MR perfusion techniques and MRS.
Results
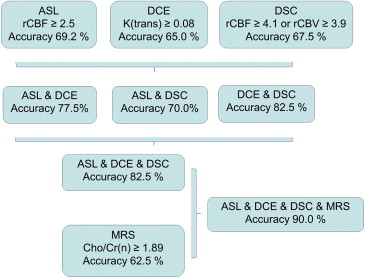
Of the 40 patients, 23 were determined to have recurrent gliomas. Differences in arterial spin labeling between the two groups were not statistically significant ( P = .063). Sensitivities and specificities for the detection of recurrent lesions in dynamic contrast-enhanced T1-weighted perfusion imaging and DSC were 61.9% and 80% transfer constant k(trans) , 77.3% and 84.6% for cerebral blood flow, and 81% and 76.9% for cerebral blood volume, respectively. Among the parameters in MRS, the ratio of choline to normalized creatine showed the best diagnostic accuracy ( P = .014; sensitivity 70%, specificity 78.6%). When considering all perfusion modalities, diagnostic accuracy could be increased to 82.5%, adding MRS to the multiparametric approach resulted in a diagnostic accuracy of 90.0%.
Conclusions
MR perfusion techniques and MRS are useful tools that enable improved differentiation between recurrent glioma and stable disease. Among the single parameters, DSC showed the best diagnostic performance. Multiparametric assessment substantially improved the ability to differentiate the two entities.
The current therapy for high-grade gliomas includes surgical resection followed by radiotherapy and chemotherapy, but tumor recurrence or progression is often seen in the course of the disease. Furthermore, up to 30% of these patients develop treatment-related injury that can mimic tumor recurrence or progression in conventional magnetic resonance imaging (MRI) . On anatomic MRI, corpus callosum involvement and multiple enhancing lesions may suggest recurrent tumor , but this is not robustly reliable. Contrast-enhanced MRI has a low specificity in the differentiation because a breakdown of the blood–brain barrier is seen in both radiation-induced necrosis and tumor recurrence. In many cases, the peritumoral territory will contain both chemoradiation injury and possible recurrent tumor . This mixed pathology makes it impossible to dichotomize patients into either tumor recurrence or pure radionecrosis. Even histological results may contain mixed pathology, and the determination whether tumor cells after radiation are viable is often difficult . Surgical biopsy samples may not be representative of the whole lesion, especially via the minimally invasive approach. Therefore, the term “stable disease” was proposed by some authors. The clinical outcome and management strategy of patients with tumor progression differ profoundly, and functional MRI is increasingly used to monitor the therapeutic effects of combined chemoradiation schemas in treating gliomas. A reliable noninvasive methodology for the differentiation between stable disease and tumor recurrence is highly needed.
In theory, the membrane turnover, the cell density, and the vasculature expression are higher in recurrent tumors than in radiation injuries . This could be measured in metabolic and hemodynamic parameters. Functional MRI enables the assessment of several parameters using different technical approaches. Perfusion techniques including arterial spin labeling (ASL), dynamic contrast-enhanced T1-weighted imaging (DCE), and dynamic susceptibility-weighted contrast-enhanced imaging (DCS) are most frequently used and enable the assessment of perfusion parameters like cerebral blood flow (CBF), cerebral blood volume (CBV), and the transfer constant between the blood plasma and extracellular space, k(trans) . However, there is no consensus regarding which technique provides the best diagnostic accuracy for the differentiation of recurrent glioma from stable disease. In addition, MR spectroscopy (MRS) enables the quantification and comparison of metabolites including choline (Cho), creatine (Cr), and N -acetylaspartate (NAA).
Get Radiology Tree app to read full this article<
Materials and methods
Patient Population
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Imaging Protocol
Get Radiology Tree app to read full this article<
ASL Imaging
Get Radiology Tree app to read full this article<
DCE Imaging
Get Radiology Tree app to read full this article<
DCS Imaging
Get Radiology Tree app to read full this article<
MRS
Get Radiology Tree app to read full this article<
Image Evaluation
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Statistical Analysis
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Results
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Table 1
Quantitative Results
Age Gender ASL DCE MR DSC MR MRS rCBF_k(trans)_ max v(e) rCBF rCBV Cho/NAA Cho/Cr NAA/Cr(n) Cho/Cr(n) Tumor recurrence 54.7 ± 15 14 Men, 9 women 2.41 ± 1.3 0.08 ± 0.06 0.27 ± 0.12 4.01 ± 2.32 3.91 ± 2.81 2.20 ± 1.55 1.66 ± 0.72 1.01 ± 0.69 1.78 ± 1.30 Stable disease 52.1 ± 10 10 Men, 7 women 1.66 ± 0.5 0.047 ± 0.02 0.15 ± 0.17 1.66 ± 1.18 1.73 ± 1.14 1.47 ± 1.57 1.30 ± 0.68 0.51 ± 0.40 0.65 ± 0.44P -value n.s. n.s. .063 .046 .10 <.01 .01 >.01 .047 .051 .014
The table summarizes the quantitative results of the comparison of the two study groups (tumor recurrence and stable disease). The parameters k(trans) , rCBF, rCBV, Cho/Cr, and Cho/Cr(n) reach statistical significance. ASL, arterial spin labeling perfusion; DCE, dynamic contrast-enhanced T1-weighted perfusion imaging; DSC, dynamic susceptibility contrast-enhanced perfusion imaging; MR, magnetic resonance; MRS, MR spectroscopy; rCBF, normalized values for cerebral blood flow; k(trans), transfer constant; rCBV, normalized values for cerebral blood volume; Cho, choline; Cr, creatine; (n), normalized, n.s., nonsignificant.
Get Radiology Tree app to read full this article<
ASL Imaging
Get Radiology Tree app to read full this article<
DCE Imaging
Get Radiology Tree app to read full this article<
DSC Imaging
Get Radiology Tree app to read full this article<
MRS
Get Radiology Tree app to read full this article<
Combined Implementation of ASL, DCE, DSC, and MRS
Get Radiology Tree app to read full this article<
Table 2
Receiver Operating Characteristic Analysis
ASL DCE MR DSC MR MRS Scoring System rCBF_k(trans)_ max rCBF rCBV Cho/Cr Cho/Cr(n) Multiparametric Cut-off 2.18 0.058 2.24 2.15 1.12 1.07 2 Sensitivity 53.9 61.9 77.3 81.0 85.0 70.0 74 Specificity 84.6 80.0 84.6 76.9 50.0 78.6 94 Accuracy 69.2 69.4 80.0 79.4 71.9 73.5 82.5 Cut-off 100% specificity >2.46 >0.075 >4.14 >3.92 >2.33 >1.89 Not applicable Accuracy (100% specificity) 69.2 65.0 61.5 61.5 58.9 62.5
The table displays the threshold values with maximum accuracy and maximum specificity in discriminating between recurrent glioma and stable disease. ASL, arterial spin labeling perfusion; DCE, dynamic contrast-enhanced T1-weighted perfusion imaging; DSC, dynamic susceptibility contrast-enhanced perfusion imaging; MR, magnetic resonance; MRS, MR spectroscopy; rCBF, normalized values for cerebral blood flow; k(trans), transfer constant; rCBV, normalized values for cerebral blood volume; Cho, choline; Cr, creatine; (n), normalized.
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Discussion
Get Radiology Tree app to read full this article<
ASL Imaging
Get Radiology Tree app to read full this article<
DCE Imaging
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
DSC Imaging
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
MRS
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Multimodality Functional Imaging
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Conclusions
Get Radiology Tree app to read full this article<
References
1. Alexiou G.A., Tsiouris S., Kyritsis A.P., et. al.: Glioma recurrence versus radiation necrosis: accuracy of current imaging modalities. J Neuro-oncol 2009; 95: pp. 1-11.
2. Brandes A.A., Tosoni A., Spagnolli F., et. al.: Disease progression or pseudoprogression after concomitant radiochemotherapy treatment: pitfalls in neurooncology. Neuro-oncology 2008; 10: pp. 361-367.
3. Mullins M.E., Barest G.D., Schaefer P.W., et. al.: Radiation necrosis versus glioma recurrence: conventional MR imaging clues to diagnosis. AJNR Am J Neuroradiol 2005; 26: pp. 1967-1972.
4. Al Sayyari A., Buckley R., McHenery C., et. al.: Distinguishing recurrent primary brain tumor from radiation injury: a preliminary study using a susceptibility-weighted MR imaging-guided apparent diffusion coefficient analysis strategy. AJNR Am J Neuroradiol 2010; 31: pp. 1049-1054.
5. Barajas R.F., Chang J.S., Segal M.R., et. al.: Differentiation of recurrent glioblastoma multiforme from radiation necrosis after external beam radiation therapy with dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging. Radiology 2009; 253: pp. 486-496.
6. Perry A., Schmidt R.E.: Cancer therapy-associated CNS neuropathology: an update and review of the literature. Acta Neuropathol 2006; 111: pp. 197-212.
7. Shah A.H., Snelling B., Bregy A., et. al.: Discriminating radiation necrosis from tumor progression in gliomas: a systematic review what is the best imaging modality?. J Neuro-oncol 2013; 112: pp. 141-152.
8. Stupp R., Mason W.P., van den Bent M.J., et. al.: Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005; 352: pp. 987-996.
9. Buxton R.B., Frank L.R., Wong E.C., et. al.: A general kinetic model for quantitative perfusion imaging with arterial spin labeling. Magn Reson Med 1998; 40: pp. 383-396.
10. Bisdas S., Naegele T., Ritz R., et. al.: Distinguishing recurrent high-grade gliomas from radiation injury: a pilot study using dynamic contrast-enhanced MR imaging. Acad Radiol 2011; 18: pp. 575-583.
11. Zwick S., Brix G., Tofts P.S., et. al.: Simulation-based comparison of two approaches frequently used for dynamic contrast-enhanced MRI. Eur Radiol 2010; 20: pp. 432-442.
12. Verma N., Cowperthwaite M.C., Burnett M.G., et. al.: Differentiating tumor recurrence from treatment necrosis: a review of neuro-oncologic imaging strategies. Neuro-oncology 2013; 15: pp. 515-534.
13. Tofts P.S., Kermode A.G.: Measurement of the blood-brain barrier permeability and leakage space using dynamic MR imaging. 1. Fundamental concepts. Magn Reson Med 1991; 17: pp. 357-367.
14. Matsusue E., Fink J.R., Rockhill J.K., et. al.: Distinction between glioma progression and post-radiation change by combined physiologic MR imaging. Neuroradiology 2010; 52: pp. 297-306.
15. Jain R., Narang J., Sundgren P.M., et. al.: Treatment induced necrosis versus recurrent/progressing brain tumor: going beyond the boundaries of conventional morphologic imaging. J Neuro-oncol 2010; 100: pp. 17-29.
16. Petcharunpaisan S., Ramalho J., Castillo M.: Arterial spin labeling in neuroimaging. World J Radiol 2010; 2: pp. 384-398.
17. Jarnum H., Steffensen E.G., Knutsson L., et. al.: Perfusion MRI of brain tumours: a comparative study of pseudo-continuous arterial spin labelling and dynamic susceptibility contrast imaging. Neuroradiology 2010; 52: pp. 307-317.
18. Wolf R.L., Detre J.A.: Clinical neuroimaging using arterial spin-labeled perfusion magnetic resonance imaging. Neurotherapeutics 2007; 4: pp. 346-359.
19. Weber M.A., Gunther M., Lichy M.P., et. al.: Comparison of arterial spin-labeling techniques and dynamic susceptibility-weighted contrast-enhanced MRI in perfusion imaging of normal brain tissue. Investig Radiol 2003; 38: pp. 712-718.
20. Ozsunar Y., Mullins M.E., Kwong K., et. al.: Glioma recurrence versus radiation necrosis? A pilot comparison of arterial spin-labeled, dynamic susceptibility contrast enhanced MRI, and FDG-PET imaging. Acad Radiol 2010; 17: pp. 282-290.
21. Narang J., Jain R., Arbab A.S., et. al.: Differentiating treatment-induced necrosis from recurrent/progressive brain tumor using nonmodel-based semiquantitative indices derived from dynamic contrast-enhanced T1-weighted MR perfusion. Neuro-oncology 2011; 13: pp. 1037-1046.
22. Tofts P.S., Brix G., Buckley D.L., et. al.: Estimating kinetic parameters from dynamic contrast-enhanced T(1)-weighted MRI of a diffusable tracer: standardized quantities and symbols. J Magn ResonI Imaging 1999; 10: pp. 223-232.
23. Fatterpekar G.M., Galheigo D., Narayana A., et. al.: Treatment-related change versus tumor recurrence in high-grade gliomas: a diagnostic conundrum–use of dynamic susceptibility contrast-enhanced (DSC) perfusion MRI. AJR Am J Roentgenol 2012; 198: pp. 19-26.
24. Hu L.S., Baxter L.C., Smith K.A., et. al.: Relative cerebral blood volume values to differentiate high-grade glioma recurrence from posttreatment radiation effect: direct correlation between image-guided tissue histopathology and localized dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging measurements. AJNR Am J Neuroradiol 2009; 30: pp. 552-558.
25. Sugahara T., Korogi Y., Tomiguchi S., et. al.: Posttherapeutic intraaxial brain tumor: the value of perfusion-sensitive contrast-enhanced MR imaging for differentiating tumor recurrence from nonneoplastic contrast-enhancing tissue. AJNR Am J Neuroradiol 2000; 21: pp. 901-909.
26. Gasparetto E.L., Pawlak M.A., Patel S.H., et. al.: Posttreatment recurrence of malignant brain neoplasm: accuracy of relative cerebral blood volume fraction in discriminating low from high malignant histologic volume fraction. Radiology 2009; 250: pp. 887-896.
27. Hu L.S., Eschbacher J.M., Heiserman J.E., et. al.: Reevaluating the imaging definition of tumor progression: perfusion MRI quantifies recurrent glioblastoma tumor fraction, pseudoprogression, and radiation necrosis to predict survival. Neuro-oncology 2012; 14: pp. 919-930.
28. Paulson E.S., Schmainda K.M.: Comparison of dynamic susceptibility-weighted contrast-enhanced MR methods: recommendations for measuring relative cerebral blood volume in brain tumors. Radiology 2008; 249: pp. 601-613.
29. Weybright P., Sundgren P.C., Maly P., et. al.: Differentiation between brain tumor recurrence and radiation injury using MR spectroscopy. AJR Am J Roentgenol 2005; 185: pp. 1471-1476.
30. Zeng Q.S., Li C.F., Zhang K., et. al.: Multivoxel 3D proton MR spectroscopy in the distinction of recurrent glioma from radiation injury. J Neuro-oncol 2007; 84: pp. 63-69.
31. Elias A.E., Carlos R.C., Smith E.A., et. al.: MR spectroscopy using normalized and non-normalized metabolite ratios for differentiating recurrent brain tumor from radiation injury. Acad Radiol 2011; 18: pp. 1101-1108.
32. Smith E.A., Carlos R.C., Junck L.R., et. al.: Developing a clinical decision model: MR spectroscopy to differentiate between recurrent tumor and radiation change in patients with new contrast-enhancing lesions. AJR Am J Roentgenol 2009; 192: pp. W45-W52.
33. Rabinov J.D., Lee P.L., Barker F.G., et. al.: In vivo 3-T MR spectroscopy in the distinction of recurrent glioma versus radiation effects: initial experience. Radiology 2002; 225: pp. 871-879.
34. Tedeschi G., Lundbom N., Raman R., et. al.: Increased choline signal coinciding with malignant degeneration of cerebral gliomas: a serial proton magnetic resonance spectroscopy imaging study. J Neurosurg 1997; 87: pp. 516-524.
35. Rock J.P., Scarpace L., Hearshen D., et. al.: Associations among magnetic resonance spectroscopy, apparent diffusion coefficients, and image-guided histopathology with special attention to radiation necrosis. Neurosurgery 2004; 54: pp. 1111-1117.
36. Rock J.P., Hearshen D., Scarpace L., et. al.: Correlations between magnetic resonance spectroscopy and image-guided histopathology, with special attention to radiation necrosis. Neurosurgery 2002; 51: pp. 912-920.
37. Lima E.C., Otaduy M.C., Tsunemi M., et. al.: The effect of paramagnetic contrast in choline peak in patients with glioblastoma multiforme might not be significant. AJNR Am J Neuroradiol 2013; 34: pp. 80-84.
![Figure 1, Example of tumor recurrence in a 53-year-old patient treated with surgery and radiation with recurrence of glioma. The image shows magnetic resonance (MR) perfusion (a–c) , MR spectroscopy (MRS) (d,f) , and postcontrast T1-weighted imaging (e) . The T1-weighted contrast-enhanced images (e) show the new enhancing lesion in the former surgery area. While arterial spin labeling perfusion (a) could not reliably differentiate tumor recurrence (normalized values for cerebral blood flow 1.6), dynamic contrast-enhanced T1-weighted perfusion imaging (b) and dynamic susceptibility contrast-enhanced perfusion imaging (c) show hyperperfusion of the lesion [quantification of perfusion values: maximum k(trans) 0.062/min and normalized values for cerebral blood volume >5], an indication of tumor recurrence. MRS supports the diagnosis due to the elevated ratio of choline in the lesion (f) to creatine in the healthy tissue (d) . The patient underwent resection, and tumor recurrence was confirmed by histopathology. The illustrations show the values in a descending red-green-blue color scale. (Color version of the figure is available online.)](https://storage.googleapis.com/dl.dentistrykey.com/clinical/ComparisonofThreeDifferentMRPerfusionTechniquesandMRSpectroscopyforMultiparametricAssessmentinDistinguishingRecurrentHighGradeGliomasfromStableDisease/0_1s20S107663321300411X.jpg)
![Figure 2, Example of stable disease in a 39-year-old patient treated with surgery and radiation. The image shows magnetic resonance (MR) perfusion (a–c) , MR spectroscopy (MRS) (d,f) , and postcontrast T1-weighted imaging (e) as well as T1-weighted imaging in two serial follow-up examinations (g,h) . The T1-weighted contrast-enhanced images (e) show the new enhancing lesion in the former surgical area. While arterial spin labeling perfusion (a) and dynamic contrast-enhanced T1-weighted perfusion imaging (b) indicate normal values [normalized values for cerebral blood flow 1.3 and maximum k(trans) 0.04], normalized values for cerebral blood volume are slightly elevated in dynamic susceptibility contrast-enhanced perfusion imaging (c) with a value of 2.2 ( arrow ). MRS supports the diagnosis of stable disease due to normal choline in the lesion (d) and normal ratio normalized to the healthy tissue (f) . The lesion seems to resolve in two serial follow-up examinations (g,h) , confirming the diagnosis of stable disease. The illustrations show the values in a descending red-green-blue color scale. (Color version of the figure is available online.)](https://storage.googleapis.com/dl.dentistrykey.com/clinical/ComparisonofThreeDifferentMRPerfusionTechniquesandMRSpectroscopyforMultiparametricAssessmentinDistinguishingRecurrentHighGradeGliomasfromStableDisease/1_1s20S107663321300411X.jpg)