Rationale and Objectives
Our goal was to evaluate the role of contrast-enhanced gray-scale transrectal ultrasound (CETRUS)-guided prostate biopsy in patients with elevated serum prostate-specific antigen (PSA) levels.
Materials and Methods
A total of 115 men (mean age, 70 years; range, 47−85) with serum PSA levels of greater than 4.0 ng/ml were assessed using gray-scale transrectal ultrasound (TRUS), power Doppler ultrasound (PDU), and CETRUS. Subsequently, these patients underwent systematic sextant transrectal biopsy and additional biopsies for positive sites on gray-scale TRUS, PDU, and CETRUS. The cancer detection rates of the three techniques were compared.
Results
Cancer was detected in 63 of the 115 patients (55%). CETRUS was positive in 50 patients, 35 of whom (70%) had prostate cancer; CETRUS had a higher sensitivity, specificity, and accuracy of 65% (41/63), 83% (43/52), and 73% (84/115), respectively. CETRUS could have saved a significant number of patients from undergoing unnecessary biopsies, compared to TRUS and PDU. However, no significant correlation was found between the Gleason score and CETRUS grade.
Conclusions
The use of CETRUS in detecting prostate cancer might reduce the number of unnecessary needle biopsies of the prostate in patients with abnormally high serum PSA levels and increase the detection rate of clinically significant prostate cancer.
Prostate cancer is one of the most notable causes of death from cancer in men. The detection of prostate cancer is generally based on digital rectal examination (DRE) and transrectal ultrasound (TRUS) findings and serum prostate-specific antigen (PSA) determination ( ). TRUS is the most commonly used imaging technique to guide the needle biopsy due to its excellent demonstration of zonal anatomy. However, multiple systematic random biopsies have been shown to overlook a large number of clinically significant carcinomas. This fact has led to a dramatic increase in the number of biopsy samples taken for the detection of localized prostate cancer ( ). There is an increasing need for improved diagnostic imaging to more accurately identify prostate cancer.
Prostate cancer tissue is associated with increased microvessel density due to the proliferation of neovessels. Microvessels in malignant tissue are smaller than those of benign prostate tissue ( ). The microvessels that proliferate in prostate cancer are below the resolution of conventional Doppler imaging; only the larger feeding vessels are visualized by this type of imaging ( ).
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Materials and methods
Patients
Get Radiology Tree app to read full this article<
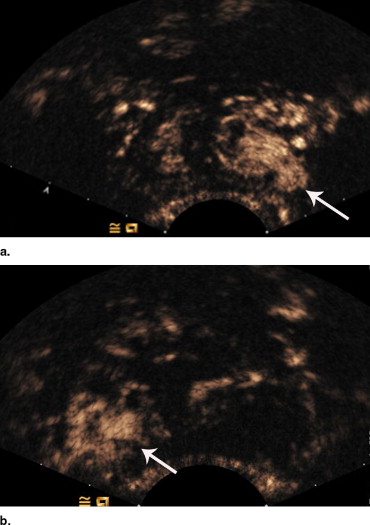
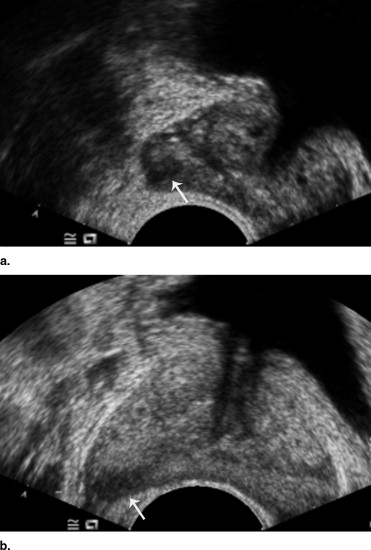
Contrast-Enhanced Transrectal Gray-scale Ultrasonography and Image Analysis
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
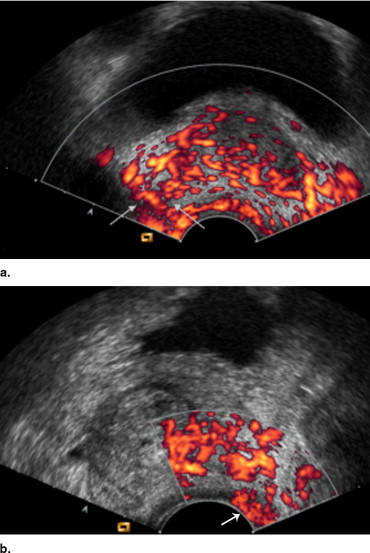
Transrectal Ultrasound-guided Biopsy
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Statistical Analysis
Get Radiology Tree app to read full this article<
Results
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Table 1
Relationship Between the Contrast-Enhanced Gray-Scale Transrectal Ultrasound (CETRUS) Grades and the Biopsy Results ( n = 115)
Histologic Finding CETRUS Grade Total (%) 0 1 2 3 Cancer positive Gleason score 8−10 3 7 12 3 25 5−7 4 7 22 4 37 2−4 1 0 0 0 1 Subtotal 8 14 34 7 63 (55) Cancer negative BPH 32 7 4 0 43 Inflammatory change 2 1 3 1 7 PIN 0 1 1 0 2 Subtotal 34 9 8 1 52 (45) Total (%) 42 23 42 8 115 (100)
BPH: benign prostatic hyperplasia; PIN: prostatic intraepithelial neoplasia.
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Table 2
Relationship Between the Power Doppler Ultrasound (PDU) Grades and the Biopsy Results ( n = 115)
Histologic Finding PDU Grade Total (%) 0 1 2 Cancer positive Gleason score 8−10 10 8 3 21 5−7 16 21 4 41 2−4 1 0 0 1 Subtotal 27 29 7 63 (55) Cancer negative BPH 32 7 1 40 Inflammatory change 2 3 5 8 PIN 0 2 0 2 Subtotal 34 12 6 52 (45) Total (%) 61 41 13 115 (100)
BPH: benign prostatic hyperplasia; PIN: prostatic intraepithelial neoplasia.
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Table 3
Comparison of Test Performance for Prostate Cancer Detection
Type of Directed Biopsy Sensitivity Specificity PPV NPV Accuracy Overall ( n = 115) Gray-scale TRUS 32/63 (51) ⁎ 30/52 (58) ⁎ 32/54 (59) 30/61 (49) 62/115 (54) PDU 36/63 (57) 34/52 (65) ⁎ 36/54 (67) 34/61 (56) 70/115 (61) CETRUS 41/63 (65) 43/52 (83) 41/50 (82) 43/65 (66) 84/115 (73) Serum PSA 4.1–10 ng/ml ( n = 36) Gray-scale TRUS 4/11 (36) 14/25 (56) 4/15 (27) 14/21 (67) 18/36 (50) PDU 5/11 (45) 16/25 (64) 5/14 (36) 16/22 (73) 21/36 (58) CETRUS 6/11 (55) 21/25 (84) 6/10 (60) 21/26 (81) 27/36 (75)
CETRUS: contrast-enhanced transrectal ultrasound; NPV: negative predictive value; PSA: prostate-specific antigen; PDU: power Doppler ultrasound; PPV: positive predictive value; TRUS: transrectal ultrasound.
Data in parentheses are percentages. Sensitivity and specificity of gray-scale TRUS and PDU were compared with those of CETRUS using the χ 2 test.
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Discussion
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Conclusion
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
References
1. Catalona W.J., Richie J.P., Ahman F.R.: A multicenter evaluation of PSA and digital rectal examination (DRE) for early detection of prostate cancer in 6,371 volunteers. J Urol 1993; 149: pp. 412-421.
2. Cooper W.H., Mosley B.R., Rutherford C.T.: Prostate cancer detection in a clinical urological practice by ultrasonography, digital rectal examination and prostate specific antigen. J Urol 1990; 143: pp. 1146-1154.
3. Loch T.: Urologic imaging for localized prostate cancer in 2007. World J Urol 2007; 25: pp. 121-129.
4. Louvar E., Littrup P.J., Goldstein A., Yu L., Sakr W., Grignon D.: Correlation of color flow in the prostate with tissue microvascularity. Cancer 1998; 83: pp. 135-140.
5. Halpern E.J.: Contrast-enhanced ultrasound imaging of prostate cancer. Rev Urol 2006; 8: pp. 29-37.
6. Wilson S.R., Burns P.N.: Microbubble contrast for radiological imaging: 2. Ultrasound Q 2006; 22: pp. 15-18.
7. Sedelaar J.P., van Leenders G.J., Hulsbergen-van de Kaa C.A., et. al.: Microvessel density: Correlation between contrast ultrasonography and histology of prostate cancer. Eur Urol 2001; 40: pp. 285-293.
8. Halpern E.J., McCue P.A., Aksnes A.K., Hagen E.K., Frauscher F., Gomella L.G.: Contrast-enhanced US of the prostate with Sonazoid: Comparison with whole-mount prostatectomy specimens in 12 patients. Radiology 2002; 222: pp. 361-366.
9. Halpern E.J., Verkh L., Forsberg F., Gomella L.G., Mattrey R.F., Goldberg B.B.: Initial experience with contrast-enhanced sonography of the prostate. AJR Am J Roentgenol 2000; 174: pp. 1575-1580.
10. Schneider M., Arditi M., Barrau M.B., et. al.: BR1: A new ultrasonographic contrast agent based on sulfur hexafluoride-filled microbubbles. Invest Radiol 1995; 30: pp. 451-457.
11. Kaps M., Legemate D.A., Ries F., et. al.: SonoVue in transcranial Doppler investigations of the cerebral arteries. J Neuroimaging 2001; 11: pp. 261-267.
12. Catalona W.J., Ramos C.G., Carvalhal G.F., Yan Y.: Lowering PSA cutoffs to enhance detection of curable prostate cancer. Urology 2000; 55: pp. 791-795.
13. Halpern E.J., Strup S.E.: Using gray-scale and color and power Doppler sonography to detect prostatic cancer. AJR Am J Roentgenol 2000; 174: pp. 623-627.
14. Halpern E.J., Frauscher F., Rosenberg M., Gomella L.G.: Directed biopsy during contrast-enhanced sonography of the prostate. AJR Am J Roentgenol 2002; 178: pp. 915-919.
15. Strohmeyer D., Frauscher F., Klauser A., et. al.: Contrast-enhanced transrectal color Doppler ultrasonography (TRCDUS) for assessment of angiogenesis in prostate cancer. Anticancer Res 2001; 21: pp. 2907-2913.
16. Tang J., Li S., Li J., et. al.: Evaluation of the effect of protamine on human prostate carcinoma PC-3m using contrast enhanced Doppler ultrasound. J Urol 2003; 170: pp. 611-614.
17. Phillips P.J.: Contrast pulse sequences (CPS): Imaging nonlinear microbubbles. Ultrasonics Symp 2001; 2: pp. 1739-1745.
18. Halpern E.J., Rosenberg M., Gomella L.G.: Prostate cancer: Contrast-enhanced US for detection. Radiology 2001; 219: pp. 219-225.
19. Miyake H., Sakai I., Harada K., Hara I., Eto H.: Prediction of potentially insignificant prostate cancer in men undergoing radical prostatectomy for clinically organ-confined disease. Int J Urol 2005; 12: pp. 270-274.
20. Arger P.H., Malkowicz S.B., VanArsdalen K.N., Sehgal C.M., Holzer A., Schultz S.M.: Color and power Doppler sonography in the diagnosis of prostate cancer: Comparison between vascular density and total vascularity. J Ultrasound Med 2004; 23: pp. 623-630.
21. Rubin M.A., Buyyounouski M., Bagiella E., et. al.: Microvessel density in prostate cancer: Lack of correlation with tumor grade, pathologic stage, and clinical outcome. Urology 1999; 53: pp. 542-547.
22. Papadopoulos I., Sivridis E., Giatromanolaki A., Koukourakis M.I.: Tumor angiogenesis is associated with MUC1 overexpression and loss of prostate-specific antigen expression in prostate cancer. Clin Cancer Res 2001; 7: pp. 1533-1538.