Rationale and Objectives
The objective of this study was to evaluate and compare contrast-enhanced subharmonic and harmonic ultrasound as tools for characterizing solid renal masses and monitoring their response to cryoablation therapy.
Materials and Methods
Sixteen patients undergoing percutaneous ablation of a renal mass provided informed consent to undergo ultrasound examinations the morning before and approximately 4 months after cryoablation. Ultrasound contrast parameters during pretreatment imaging were compared to biopsy results obtained during ablation ( n = 13). Posttreatment changes were evaluated by a radiologist and compared to contrast-enhanced magnetic resonance imaging (MRI)/computed tomography (CT) follow-up.
Results
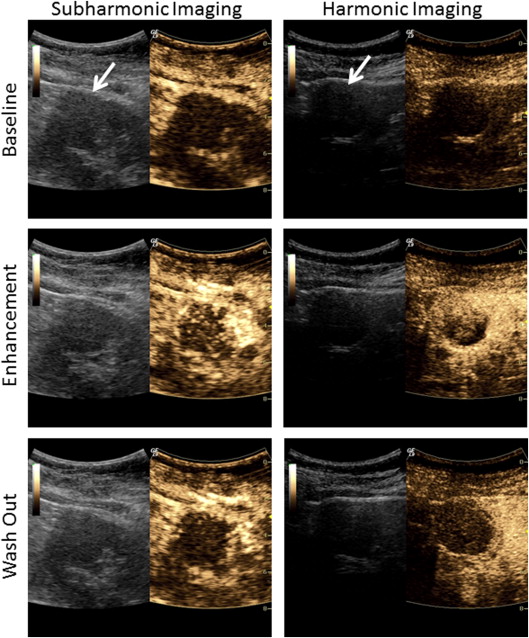
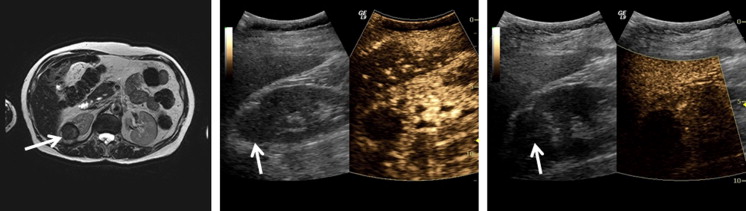
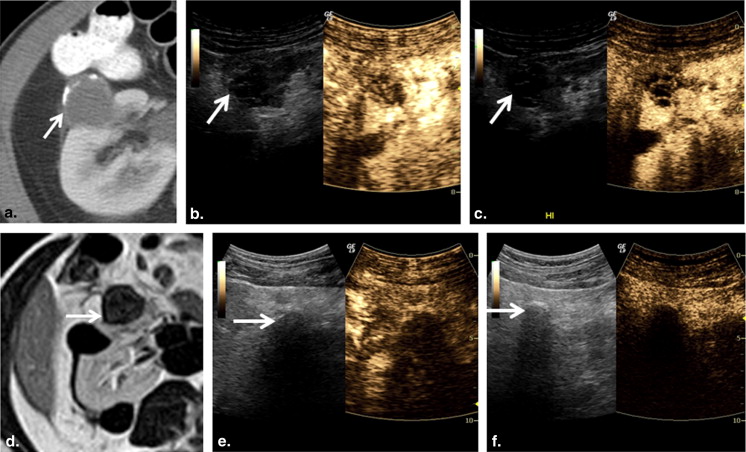
All masses initially showed heterogeneous enhancement with both subharmonic and harmonic ultrasound. Early contrast washout in the mass relative to the cortex was observed in 6 of 9 malignant and 0 of 4 benign lesions in subharmonic mode and 8 of 9 malignant and 1 of 4 benign lesions in harmonic imaging. In cases where the lesion was adequately visualized at follow-up ( n = 12), subharmonic and harmonic ultrasound showed accuracies of 83% and 75%, respectively, in predicting treatment outcome. Although harmonic imaging showed less overall error, no significant differences ( P > .29) in ablation cavity volumes were observed between MRI/CT and either contrast-imaging mode.
Conclusions
Subharmonic and harmonic contrast-enhanced ultrasound may be a safe and accurate imaging alternative for characterizing renal masses and evaluating their response to cryoablation therapy. Although subharmonic imaging was more accurate in detecting effective cryoablation, harmonic imaging was superior in quantifying ablation cavity volumes.
Renal cell carcinoma (RCC) is becoming increasingly common with 63,920 new cases and 13,860 deaths reported in the United States in 2014 . Detection rates of RCC have doubled over the past 50 years, with nearly half of reported cases being detected incidentally during magnetic resonance imaging (MRI) or computed tomography (CT) imaging . Although surgical extirpation remains the standard of care, ablative therapy is playing a larger role in the treatment of poor surgical candidates and also can preserve renal function . Ablation of RCC can be performed laparoscopically or percutaneously. However, percutaneous ablation has been associated with shorter anesthesia times, fewer probes, shorter hospital stays, lower hospital charges, and quicker recovery relative to laparoscopic approaches . Of the thermal ablation modalities, cryoablation is often preferred because it creates an ice ball during ablation which can be tracked by CT guidance during the session. Cryoablation is also significantly less painful than heat-based techniques such as microwave and radiofrequency ablation, thereby avoiding the use of general anesthesia in many patients .
Protocols for cryoablation follow-up imaging vary but mainly rely on contrast-enhanced CT or MRI 1–6 months after procedure to evaluate effective ablation based on a lack of vascularity within the mass and a reduction in tumor volume . At our institution, contrast-enhanced CT or MRI after 3–4 months represents the clinical standard, as earlier imaging has been shown to result in false positives because of artifacts associated with postablation inflammation . Although well validated in evaluating cryoablation outcomes, CT and MRI contrast materials have been associated with nephrotoxicity (iodinated contrast) and nephrogenic systemic fibrosis (gadolinium-based contrast) and are not well suited for a patient population with compromised renal function. Thus, an imaging technique which could effectively evaluate cryoablation outcomes without the associated nephrotoxicity would be beneficial in this patient population.
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Materials and methods
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Ablation Volume=43π×Length2×Width2×Height2 Ablation Volume
=
4
3
π
×
Length
2
×
Width
2
×
Height
2
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Results
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Discussion
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Acknowledgment
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
References
1. American Cancer Society: Cancer facts & figures 2014.2014.American Cancer SocietyAtlanta, GA
2. Venkateson A.M., Wood B.J., Gervais D.A.: Percutaneous ablation in the kidney. Radiology 2011; 261: pp. 375-391.
3. Levinson A.W., Su L.M., Agarwal D., et. al.: Long-term oncological and overall outcomes or percutaneous radio frequency ablation in high risk surgical patients with a solitary mass. J Urol 2008; 180: pp. 499-504.
4. Bandi G., Hedican S.P., Nakada S.Y.: Current practice patterns in the use of ablation technology for the management of small renal masses at academic centers in the United States. Urology 2008; 71: pp. 113-117.
5. Hinshaw J.L., Shadid A.M., Nakada S.Y., et. al.: Comparison of percutaneous and laparoscopic cyroablation for the treatment of solid renal masses. AJR J Roentgenol 2008; 191: pp. 1159-1168.
6. Allaf M.E., Varkarakis I.M., Bhayani S.B., et. al.: Pain control requirements for percutaneous ablation of renal tumors: cyroablation versus radiofrequency ablation-initial observations. Radiology 2005; 237: pp. 366-370.
7. Gervais D.A., Arellano R.S., McGovern F.J., et. al.: Radiofrequency ablation of renal cell carcinoma II lessons learned with ablation of 100 tumors. AJR Am J Roentgenol 2005; 188: pp. 72-80.
8. Zagoria R.J.: Imaging-guided radiofrequency ablation of renal masses. Radiographics 2004; 24: pp. S59-S71.
9. Thumar A.B., Trabulsi E.J., Lallas C.D., et. al.: Thermal ablation of renal cell carcinoma: triage, treatment, and follow-up. J VascInterv Radiol 2010; 21: pp. S233-S241.
10. 12/22/2006. updated 5/23/2007
11. Goldberg B.B., Raichlen J.S., Forsberg F.: Ultrasound contrast agents: basic principles and clinical applications.2001.Martin Dunitz Ltd.London
12. Wells P.N., Liang H.D., Young T.P.: Ultrasonic imaging technologies in perspective. J Med Eng Technol 2011; 35: pp. 289-299.
13. Shankar P.M., Dala Krishan P., Newhouse V.L.: Advantages of subharmonic over second harmonic backscatter for contrast-to-tissue echo enhancement. Ultrasound Med Biol 1998; 24: pp. 395-399.
14. Shankar P.M., Krishna P., Newhouse V.L.: Subharmonic backscattering from ultrasound contrast agents. J Acoust Soc Am 1999; 106: pp. 2104-2110.
15. Forsberg F., Shi W.T., Goldberg B.B.: Subharmonic imaging of contrast agents. Ultrasonics 2000; 38: pp. 93-104.
16. Chomas J., Dayton P., May D., et. al.: Nondestructive subharmonic imaging. IEEE Trans Ultrason Ferroelectr Freq Control 2002; 49: pp. 883-892.
17. Goertz D.E., Frijlink M.E., de Jong N., et. al.: High frequency nonlinear scattering from a micrometer to submicrometer sized lipid encapsulared contrast agent. Ultrasound Med Biol 2006; 32: pp. 569-577.
18. Goertz D.E., Frijlin M.E., Tempel D., et. al.: Subharmonic contrast intravascular ultrasound for vasa vasorum imaging. Ultrasound Med Biol 2007; 33: pp. 1859-1872.
19. Helfield B.L., Cherin E., Foster F.S., et. al.: Investigating the subharmonic response of individual phospholipid encapsulated microbubbles at high frequencies: a comparitive study of five agents. Ultrasound Med Biol 2012; 38: pp. 846-863.
20. Eisenbrey J.R., Sridharan A., Machado P., et. al.: 3D subharmonic imaging in vitro and in vivo. Acad Radiol 2012; 19: pp. 732-739.
21. Helfield B.L., Leung B.Y., Goertz D.E.: The influence of compliant boundary proximity on the fundamental and subharmonic emissions from individual microbubbles. J Acoust Soc Am 2014; 136: pp. EL40-EL46.
22. Forsberg F., Piccoli C.W., Merton D.A., et. al.: Breast lesions: imaging with contrast-enhanced subharmonic US—initial experience. Radiology 2007; 244: pp. 718-726.
23. Eisenbrey J.R., Dave J.K., Halldorsdottir V.G., et. al.: Simultaneous grayscale and subharmonic ultrasound imaging on a modified commercial scanner. Ultrasonics 2011; 51: pp. 890-897.
24. Johnson D.B., Duchene D.A., Taylor G.D., et. al.: Contrast-enhanced ultrasound evaluation of radiofrequency ablation of the kidney: reliable imaging of the thermolesion. J Endourol 2005; 19: pp. 248-252.
25. Hoeffel C., Pousset M., Timsit M.O., et. al.: Radiofrequency ablation of renal tumours: diagnostic accuracy of contrast-enhanced ultrasound for early detection of residual tumour. Eur Radiol 2010; 20: pp. 1812-1821.
26. Kong W.T., Zhang W.W., Guo H.Q., et. al.: Application of contrast-enhanced ultrasonography after radiofrequency ablation for renal cell carcinoma: is it sufficient for assessment of therapeutic response?. Abdom Imaging 2011; 36: pp. 342-347.
27. Li X., Liang P., Yu J., et. al.: Role of contrast-enhanced ultrasound in evaluating the efficiency of ultrasound guided percutaneous microwave ablation in patients with renal cell carcinoma. Radiol Oncol 2013; 47: pp. 398-404.
28. Wink M.H., Lagerveld B.W., Laguna M.P., et. al.: Cryotherapy for renal cell cancer: diagnosis, treatment, and contrast-enhanced ultrasonography for follow-up. J Endourol 2006; 20: pp. 456-458.
29. Wink M.H., Laguna M.P., Lagerveld B.W., et. al.: Contrast-enhanced ultrasonography in the follow-up of cryoablation of renal tumours: a feasibility study. BJU Int 2007; 99: pp. 1371-1375.
30. Zhu Q., Shimizu T., Endo H., et. al.: Assessment of renal cell carcinoma after cryoablation using contrast-enhanced gray scale ultrasound: a case series. Clin Imaging 2005; 29: pp. 102-108.
31. Zeccolini G., Del Biondo D., Cicero C., et. al.: Comparison of contrast-enhanced ultrasound scan (CEUS) and MRI in the follow-up of cryoablation for small renal tumors. Experience on 25 cases. Urologia 2014; 81: pp. 1-8.
32. Halldorsdottir V.G., Dave J.K., Leodore L.M., et. al.: Subharmonic contrast microbubble signals for noninvasive pressure estimation under static and dynamic flow conditions. Ultrason Imaging 2011; 33: pp. 153-164.
33. Barr R.G.: Off-label use of ultrasound contrast agents for abdominal imaging in the United States. J Ultrasound Med 2013; 32: pp. 7-12.
34. Barr R.G., Peterson C., Hindi A.: Evaluation of indeterminate renal masses with contrast-enhanced US: a diagnostic performance study. Radiology 2014; 271: pp. 133-142.
35. Shellock F.G., Spinazzi A.: MRI safety update 2008: part 1, MRI contrast agents and nephrogenic systemic fibrosis. AJR Am J Roentgenol 2008; 191: pp. 1129-1139.
36. Schräder R.: Contrast material-induced renal failure: an overview. J Interv Cardiol 2005; 18: pp. 417-423.
37. Wei K., Mulvagh S.L., Carson L., et. al.: The safety of Definity and Optison for ultrasound image enhancement: a retrospective analysis of 78,383 administered contrast doses. J Am Soc Echocardiogr 2008; 21: pp. 1202-1206.
38. Weiss R.D., Ahmad M., Villanueva F., et. al.: CaRES (contrast echocardiography registry for safety surveillance): a new prospective multicenter study to evaluate the safety of the ultrasound contrast agent definity in clinical practice. J Am Soc Echocardiogr 2012; 25: pp. 790-795.
39. Eisenbrey J.R., Shaw C.M., Lyshchik A., et. al.: Characterization of renal masses with harmonic and subharmonic contrast-enhanced ultrasound. Proc IEEE Ultrason Symp 2014; pp. 193-196.
40. Trabulsi E.J., Eisenbrey J.R., Machado P., et. al.: Contrast-enhanced harmonic and subharmonic ultrasound evaluation of renal mass cryoablation. Mid-Atlantic Section of the American Urological Association Annual Meeting.2014.
41. Eisenbrey J.R., Machado P., Shaw C.M., et. al.: Evaluation of renal mass cryoablation with contrast-enhanced harmonic and subharmonic ultrasound: preliminary results and dosage optimization. [Abstract] J Ultrasound Med 2014; 33: pp. S11.