Rationale and Objectives
We compared contrast-enhanced T1-weighted magnetic resonance (MR) imaging of the brain using different types of data acquisition techniques: periodically rotated overlapping parallel lines with enhanced reconstruction (PROPELLER, BLADE) imaging versus standard k-space sampling (conventional spin-echo pulse sequence) in the unsedated pediatric patient with focus on artifact reduction, overall image quality, and lesion detectability.
Materials and Methods
Forty-eight pediatric patients (aged 3 months to 18 years) were scanned with a clinical 1.5-T whole body MR scanner. Cross-sectional contrast-enhanced T1-weighted spin-echo sequence was compared to a T1-weighted dark-fluid fluid-attenuated inversion-recovery (FLAIR) BLADE sequence for qualitative and quantitative criteria (image artifacts, image quality, lesion detectability) by two experienced radiologists. Imaging protocols were matched for imaging parameters. Reader agreement was assessed using the exact Bowker test.
Results
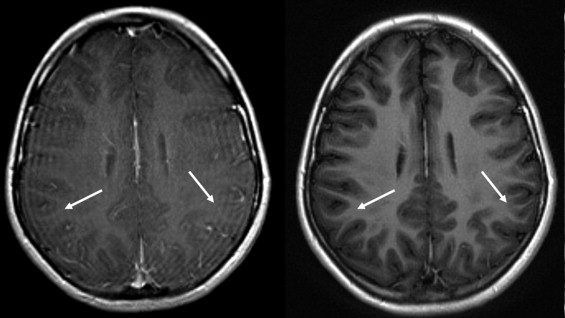
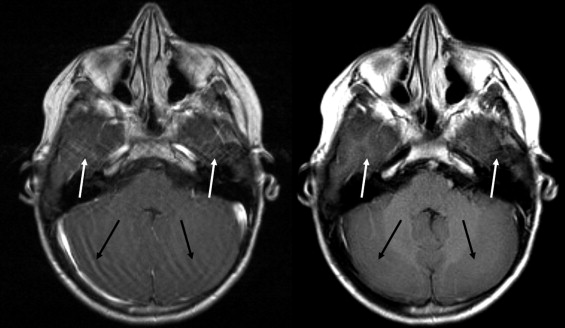
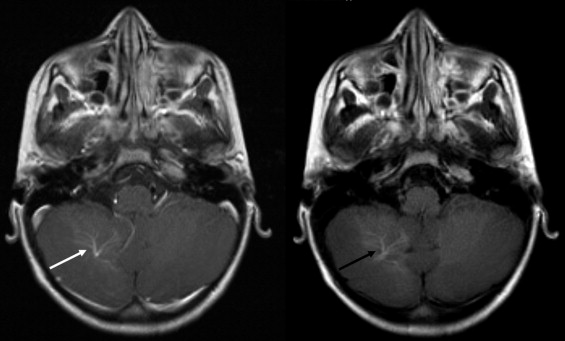
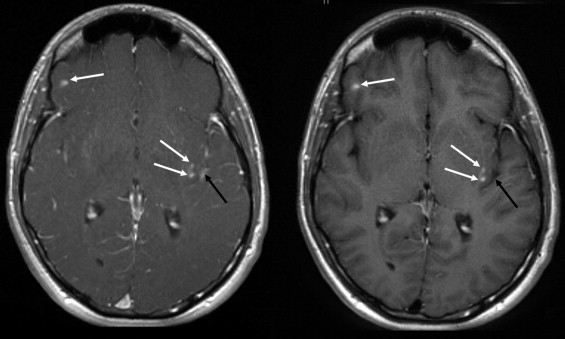
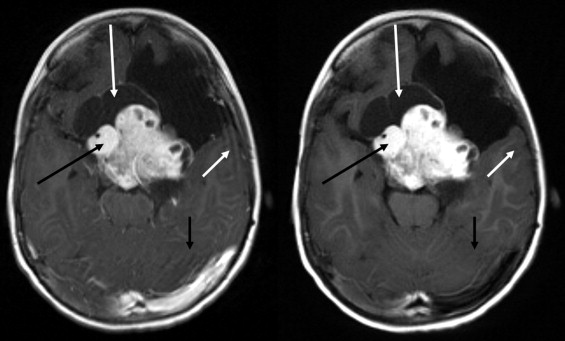
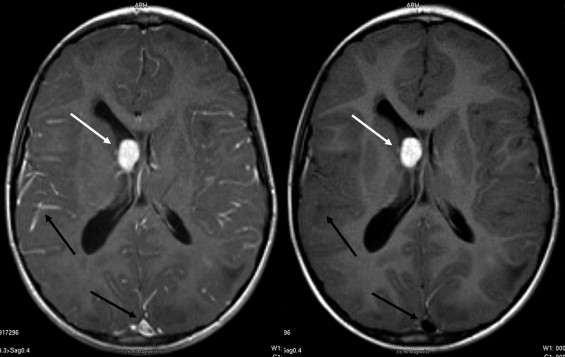
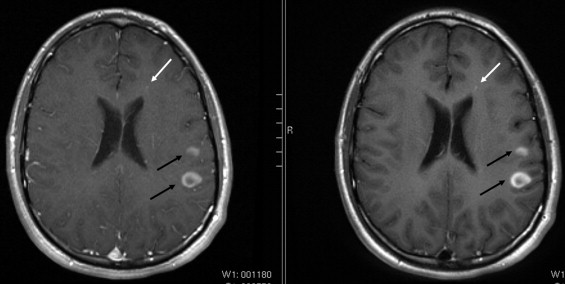
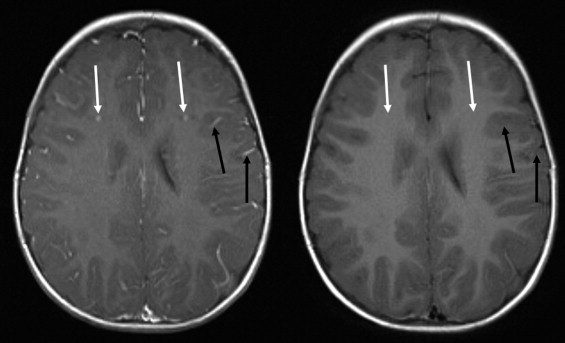
BLADE images showed significantly less pulsation and motion artifacts than the standard T1-weighted spin-echo sequence scan. BLADE images showed statistically significant lower signal-to-noise ratio but higher contrast-to-noise ratios with superior gray-white matter contrast. All lesions were demonstrated on FLAIR BLADE imaging, and one false-positive lesion was visible in spin-echo sequence images.
Conclusion
BLADE MR imaging at 1.5 T is applicable for central nervous system imaging of the unsedated pediatric patient, reduces motion and pulsation artifacts, and minimizes the need for sedation or general anesthesia without loss of relevant diagnostic information.
Magnetic resonance imaging (MRI) is a well-established imaging technique and the method of choice in the evaluation of central nervous system disorders ( ). Contrast-enhanced (CE) MRI allows classification of morphologic changes; whereas CE T1-weighted (T1w) imaging is the gold standard for displaying pathologic contrast agent uptake ( ). T1w spin-echo (SE) images are widely used to study anatomic detail and pathologic abnormalities of the brain; but this technique does not provide sufficient contrast to differentiate white matter from gray matter ( ). The quality of anatomic brain images can further be degraded by artifacts; such as subject motion ( ); the unsedated pediatric patient is particularly prone to movement. Many technical approaches to reduce motion artifacts have been reported ( ). MRI with periodically rotated overlapping parallel lines with enhanced reconstruction (PROPELLER) proposed by Pipe ( ) offers an alternative approach to imaging in the unsedated child. Almost all published data on the use of PROPELLER MRI technique at 1.5 T used T2w or diffusion weighted imaging (DWI) techniques. Recently published data showed that the T1w FLAIR BLADE technique seems to be capable of replacing T1w SE technique; at least for axial postcontrast imaging to detect brain metastases in the adult ( ). To evaluate the effect of this technique on lesion detectability; imaging artifacts; and overall image quality in the unsedated pediatric patient; which potentially produce more artifacts than adults and have varying T1 times due to immature brain parencyhma; we compared the CE T1w SE technique with a CE T1w FLAIR BLADE sequence in the transverse plane for brain MRI.
Materials and methods
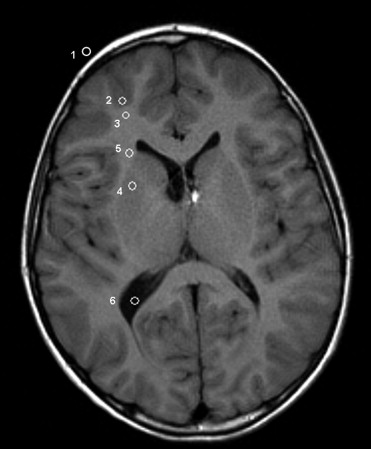
Forty-eight nonsedated pediatric patients (male/female: 26/22; age range, 3 months to 18 years; mean age, 8 years 10 months) with 40 contrast material–uptaking lesions and one developmental venous anomaly (DVA) were examined with a routine brain MRI protocol. These consisted of lesions found in acute disseminated encephalomyelitis (ADEM, n = 11), acute lymphatic leukemia ( n = 12), astrocytoma ( n = 2), and giant cell astrocytoma in patients with a history of neurofibromatosis ( n = 15) ( Table 1 ). Lesions suspicious for malignancy were proven histologically after MRI, and all other lesions were confirmed with follow-up examinations within to 6 months. Pediatric patients younger than 2 years were first fed and then placed on the scanner. All patients’ heads were fixed with the help of commercially available cushions placed on both sides of the head within the coil. Written informed consent from parents was obtained, and the study was approved by the institutional review board.
Table 1
Lesions Detected
Lesions_n_ Giant cell astrocytoma 15 ADEM 11 ALL 12 Astrocytoma 2 DVA 1 Total 41
ALL: acute lymphatic leukaemia; DVA: developmental venous anomaly.
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Results
Get Radiology Tree app to read full this article<
Qualitative Assessment
Get Radiology Tree app to read full this article<
Motion artifact
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Pulsation artifacts
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Overall imaging artifact
Get Radiology Tree app to read full this article<
Anatomic contrast
Get Radiology Tree app to read full this article<
Overall image quality/preference of data
Get Radiology Tree app to read full this article<
Lesion detection
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Blood Vessels
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Quantitative Assessment
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Table 2
Distribution of Mean Signal Intensity in Region of Interest (ROI) for Both Sequences
Region Sequence Mean STD_P_ -Value ⁎ ROI1 BLADE 21.79 5.48 SE 13.69 2.96 <.0001 ROI2 BLADE 300.94 37.85 SE 305.15 39.64 0.2033 ROI3 BLADE 401.34 52.89 SE 376.71 50.92 <.0001 ROI4 BLADE 365.90 42.88 SE 379.44 46.82 0.0002 ROI5 BLADE 413.91 54.37 SE 399.07 54.41 0.0010 ROI6 BLADE 99.44 25.53 SE 169.97 27.95 <.0001
SE: spin echo; STD: standard deviation.
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Table 3
Distribution of Signal-to Noise Ratios (SNRs) of Different Regions of Interest (ROI) in Comparison of Both Sequences
SNR Sequence Mean STD_P_ -Value ⁎ GM (ROI2) BLADE 33.48 10.82 SE 51.79 14.19 <.0001 GM (ROI4) BLADE 40.88 13.90 SE 64.45 17.43 <.0001 WM (ROI3) BLADE 44.84 14.50 SE 64.65 16.79 <.0001 WM (ROI5) BLADE 46.10 14.18 SE 66.43 20.29 <.0001 CSF (ROI6) BLADE 11.28 5.22 SE 29.10 8.93 <.0001
CSF: cerebrospinal fluid; GM: gray matter; SE: spin echo; STD: standard deviation; WM: white matter.
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Table 4
Distribution of Contrast-to-Noise Ratio (CNR) of Different Regions of Interest (ROI) in Comparison of Both Sequences
CNR Sequence Mean STD_P_ -Value ⁎ GM/WM (ROI2) BLADE 11.06 4.21 SE 12.21 4.71 0.1531 GM/WM (ROI4) BLADE 5.60 2.56 SE 4.05 2.46 0.0015 GM/CSF (ROI2) BLADE 22.60 7.38 SE 22.96 7.27 0.7687 GM/CSF (ROI4) BLADE 30.34 10.25 SE 35.45 10.54 0.0086 WM/CSF (ROI3) BLADE 32.96 11.03 SE 34.91 10.28 0.3307 WM/CSF (ROI5) BLADE 33.66 10.89 SE 38.36 11.39 0.0308 Lesion/Surrounding Parenchyma BLADE 37.92 29.46 SE 27.68 22.66 0.0140
CSF: cerebrospinal fluid; GM: gray matter; SE: spin echo; STD: standard deviation; WM: white matter.
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Discussion
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Study Limitations
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Conclusion
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
References
1. Ba-Ssalamah A., Schick S., Heimberger K., et. al.: Ultrafast magnetic resonance imaging of the brain. Magn Reson Imaging 2000; 18: pp. 237-243.
2. Essig M., Deimling M., Hawighorst H., et. al.: Assessment of cerebral gliomas by a new dark fluid sequence, high intensity reduction (HIRE): A preliminary study. J Magn Reson Imaging 2000; 11: pp. 506-517.
3. Hori M., Okubo T., Uozumi K., et. al.: T1-weighted fluid-attenuated inversion recovery at low field strength: A viable alternative for T1-Weighted Intracranial Imaging. Am J Neuroradiol 2003; 24: pp. 648-651.
4. Howarth C., Hutton C., Deichmann R.: Improvement of the image quality of T1-weighted anatomical brain scans. NeuroImage 2005; 29: pp. 930-937.
5. Murphy K.J., Brunberg J.A.: Adult claustrophobia, anxiety and sedation in MRI. Magn Reson Imaging 1997; 15: pp. 51-54.
6. Forbes K.P., Pipe J.G., Karis J.P., et. al.: Brain imaging in the unsedated paediatric patient: Comparison of periodically rotated overlapping parallel lines with enhanced reconstruction and single-shot fast spin-echo sequences. Am J Neuroradiol 2003; 24: pp. 794-798.
7. Glover G.H., Pauly J.M.: Projection reconstruction techniques for reduction of motion effects in MRI. Magn Reson Med 1992; 28: pp. 275-289.
8. Ehman R.L., Felmlee J.P.: Adaptive technique for high-definition MR imaging of moving structures. Radiology 1989; 173: pp. 255-263.
9. Sachs T.S., Meyer C.H., Irarrazabal P., et. al.: The diminishing variance algorithm for real-time reduction of motion artefacts in MRI. Magn Reson Med 1995; 34: pp. 412-422.
10. Liao J.R., Pauly J.M., Brosnan T.J., et. al.: Reduction of motion artefacts in cine MRI using variable-density spiral trajectories. Magn Reson Med 1997; 37: pp. 569-575.
11. Pipe J.G.: Motion correction with PROPELLER MR IMAGING: Application to head motion and free-breathing cardiac imaging. Magn Reson Med 1999; 42: pp. 963-969.
12. Naganawa S., Satake H., Iwano S., et. al.: Contrast-enhanced MR imaging of the brain using T1-weighted FLAIR with BLADE compared with a conventional spin-echo sequence. Eur Radiol 2008; 18: pp. 337-342.
13. Schultz C.L., Alfidi R.J., Nelson A.D., et. al.: The effect of motion on two-dimensional Fourier transformation magnetic resonance images. Radiology 1984; 152: pp. 117-121.
14. Ehman R.L., McNamara M.T., Brasch R.C., et. al.: Influence of physiologic motion on the appearance of tissue in MR images. Radiology 1986; 159: 777–182
15. Constable R.T., Smith R.C., Gore J.C.: Signal to noise and contrast in fast spin-echo (FSE) and inversion recovery FSE imaging. J Comput Assist Tomogr 1992; 16: pp. 41-47.
16. Wintersperger B.J., Runge V.M., Biswas J., et. al.: Brain magnetic resonance imaging at 3 Tesla using BLADE compared with standard rectilinear data sampling. Invest Radiol 2006; 41: pp. 586-592.
17. Forbes K.P., Pipe J.G., Bird C.R., et. al.: PROPELLER MRI: Clinical testing of a novel technique for quantification and compensation of head motion. J Magn Reson Imaging 2001; 14: pp. 215-222.