Rationale and Objectives
To demonstrate the correlation of proinflammatory cytokines (PCs), intercellular adhesion molecule (sICAM-1), tumor necrosis factor-α (TNF-α), and interleukin-1β (IL-1β) in CSF of tuberculous meningitis (TBM) patients with magnetic resonance imaging (MRI) including diffusion tensor imaging (DTI) and also to look for the changes in imaging parameters after antitubercular treatment (ATT) in these patients.
Materials and Methods
Forty patients with TBM (median age, 27.7 years) and 30 age-/sex-matched controls were included in this study. PCs were quantified from the CSF of TBM patients at the time of hospital admission (baseline). MRI including DTI was performed at the time of baseline study and 6 months after ATT.
Results
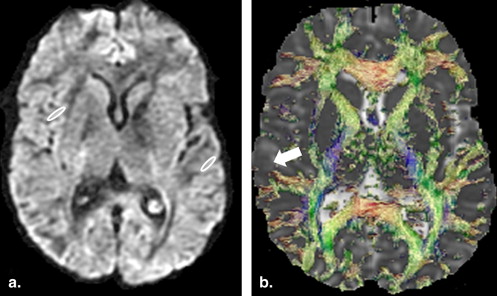
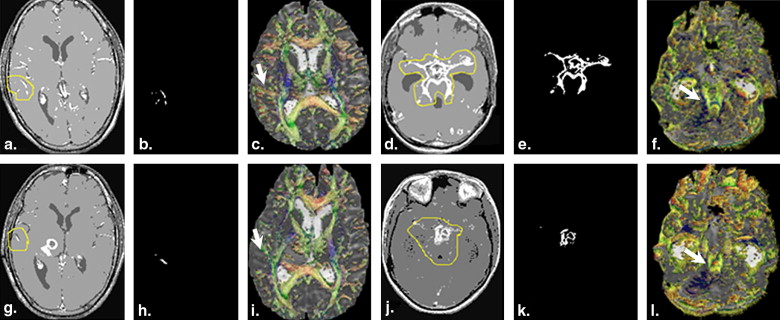
Significant positive correlation of PCs with fractional anisotropy (FA) values and post-contrast signal intensity (PCSI) collected from cerebral cortical regions was observed in TBM patients. A significant positive correlation of FA values with PCSI was also observed at both time points in patient groups. At baseline study significantly high FA values were observed in patients compared to controls. Significantly decreased FA values and PCSI were observed in the patients after 6 months of ATT compared to the baseline study.
Conclusions
Results of this study suggest that the DTI-derived anisotropy have the potential to delineate meningeal inflammation and it may be used in assessment of therapeutic response in TBM patients as an additional method to conventional imaging.
Tuberculous meningitis (TBM) is the most devastating form of tuberculosis and is associated with high morbidity and mortality worldwide . Its prevalence is increasing globally and is becoming a major problem in the Western world because of the increasing number of immunocompromised and to multidrug-resistant patients with tuberculosis TBM is fatal in 25% of adults and causes neurological sequelae in 25% of survivors even after the administration of modern chemotherapy .
In TBM, bacilli seed to brain parenchyma or meninges to form Rich foci. These foci rupture with release of bacilli in to subarachnoid space and stimulates release of proinflammatory cytokines (PCs) (eg, tumor necrosis factor-α [TNF-α], interleukin-1β [IL-1β]) and also increases the concentration of white blood cells in cerebrospinal fluid (CSF) . TNF-α has been shown to be associated with the development of TBM pathology in vivo, including tissue necrosis and cachexia . It is produced in response to the bacterial pathogen and mediates the upregulation of adhesion molecules such as selectins, intercellular adhesion molecules (ICAMs), and vascular cell adhesion molecules . IL-1β and TNF-α, are also responsible for the development of granuloma in TBM patients . In experimental bacterial meningitis, TNF-α has been shown to be responsible for the blood-brain barrier permeability as well as leukocytosis . Tsenova et al observed that TNF-α promote the progression of TBM in murine model .
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Materials and methods
Subjects
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
CSF Culture
Get Radiology Tree app to read full this article<
Enzyme-linked Immunosorbent Assay
Get Radiology Tree app to read full this article<
Imaging Protocol
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
PCSI and DTI Data Processing and Quantification
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Statistical Analysis
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Results
Laboratory Examinations
Get Radiology Tree app to read full this article<
Qualitative Image Analysis
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Quantitative Analysis
Get Radiology Tree app to read full this article<
Correlation among PCs, DTI Metrics, and PCSI at the Time of Baseline Study
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Comparison of DTI Metrics in TBM Patients with Healthy Controls
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Comparison of DTI Metrics in TBM Patients between Baseline and Follow-up Study
Get Radiology Tree app to read full this article<
Comparison of PCSI in TBM Patients between Baseline and Follow-up Study
Get Radiology Tree app to read full this article<
Discussion
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
References
1. Smith S., Jacobs R.F., Wilson C.B.: Immunobiology of childhood tuberculosis: a window on the ontogeny of cellular immunity. J Pediatr 1997; 131: pp. 16-26.
2. Bernaerts A., Vanhoenacker F.M., Parizel P.M., et. al.: Tuberculosis of the central nervous system: overview of neuroradiological findings. Eur Radiol 2003; 13: pp. 1876-1890.
3. Rich A.R., Mccordock H.A.: The pathogenesis of tuberculous meningitis. Bull Johns Hopkins Hosp 1933; 52: pp. 5-37.
4. Mustafa M.M., Lebel M.H., Ramilo O., et. al.: Correlation of interleukin-1 beta and cachectin concentrations in cerebrospinal fluid and outcome from bacterial meningitis. J Pediatr 1989; 115: pp. 208-213.
5. Beutler B., Cerami A.: The biology of cachectin/TNF-a primary mediator of the host response. Annu Rev Immunol 1989; 7: pp. 625-635.
6. Ramilo O., Sáez-Llorens X., Mertsola J., et. al.: Tumor necrosis factor alpha/cachectin and interleukin 1 beta initiate meningeal inflammation. J Exp Med 1990; 172: pp. 497-507.
7. Tsenova L., Sokol K., Freedman V.H., et. al.: A combination of thalidomide plus antibiotics protects rabbits from mycobacterial meningitis-associated death. J Infect Dis 1998; 177: pp. 1563-1572.
8. Chang K.H., Han M.H., Roh J.K., et. al.: Gd-DTPA-enhanced MR imaging of the brain in patients with meningitis: comparison with CT. AJNR Am J Neuroradiol 1990; 11: pp. 69-76.
9. Woff S.D., Balaban R.S.: Magnetization transfer contrast (MTC) and tissue water proton relaxation in vivo. Magn Reson Med 1989; 10: pp. 135-144.
10. Gupta R.K., Kathuria M.K., Pradhan S., et. al.: Magnetization transfer MR imaging in CNS tuberculosis. AJNR Am J Neuroradiol 1999; 20: pp. 867-875.
11. Mehta R.C., Bruce P.G., Enzmann D.R.: Magnetization transfer MR of the normal adult brain. AJNR Am J Neuroradiol 1995; 16: pp. 2085-2091.
12. Basser P.J., Jones D.K.: Diffusion-tensor MRI: theory, experimental design and data analysis—a technical review. NMR Biomed 2002; 15: pp. 456-467.
13. Beaulieu C.: The basis of anisotropic water diffusion in the nervous system—a technical review. NMR Biomed 2002; 15: pp. 435-445.
14. Hasan K.M., Parker D.L., Alexander A.L.: Comparison of optimization procedures for diffusion-tensor encoding directions. J Magn Reson Imaging 2001; 13: pp. 769-780.
15. Hasan K.M., Alexander A.L., Narayana P.A.: Does fractional anisotropy have better noise immunity characteristics than relative anisotropy in diffusion tensor MRI? An analytical approach. Magn Reson Med 2004; 51: pp. 413-417.
16. Pfefferbaum A., Sullivan E.V.: Disruption of brain white matter microstructure by excessive intracellular and extracellular fluid in alcoholism: evidence from diffusion tensor imaging. Neuropsychopharmacology 2005; 30: pp. 423-432.
17. Trivedi R., Malik G.K., Gupta R.K., et. al.: Increased anisotropy in neonatal meningitis: an indicator of meningeal inflammation. Neuroradiology 2007; 49: pp. 767-775.
18. Yadav A., Malik G.K., Trivedi R., et. al.: Correlation of CSF neuroinflammatory molecules with leptomeningeal cortical subcortical white matter fractional anisotropy in neonatal meningitis. Mag Reson Imaging 2009; 27: pp. 214-221.
19. Katrak S.M., Shembalkar P.K., Bijwe S.R., et. al.: The clinical, radiological and pathological profile of tuberculous meningitis in patients with and without human immunodeficiency virus infection. J Neurol Sci 2000; 181: pp. 118-126.
20. Shankar P., Manjunath N., Mohan K.K., et. al.: Rapid diagnosis of tuberculous meningitis by polymerase chain reaction. Lancet 1991; 337: pp. 5-7.
21. Mathews V.P., Kuharik M.A., Edwards M.K., et. al.: Gd-DTPA-enhanced MR imaging of experimental bacterial meningitis: evaluation and comparison with CT. AJR Am J Roentgenol 1989; 152: pp. 131-136.
22. Haris M., Gupta R.K., Husain M., et. al.: Assessment of therapeutic response in brain tuberculomas using serial dynamic contrast-enhanced MRI. Clin Radiol 2008; 63: pp. e562-e564.
23. Hasan K.M., Parker D.L., Alexander A.L.: Comparison of gradient encoding schemes for diffusion-tensor MRI. J Magn Reson Imag 2001; 13: pp. 769-780.
24. Saksena S., Rai V., Saraswat V.A., et. al.: Cerebral diffusion tensor imaging and in vivo proton magnetic resonance spectroscopy in patients with fulminant hepatic failure. J Gastroenterol Hepatol 2008; 23: pp. e111-e119.
25. Agarwal V., Kumar M., Singh J.K., et. al.: Diffusion tensor anisotropy magnetic resonance imaging: a new tool to assess synovial inflammation. Rheumatology 2009; 48: pp. 378-382.
26. Gupta R.K., Hasan K.M., Mishra A.M., et. al.: High fractional anisotropy in brain abscess verses other cystic intra cranial lesions. AJNR Am J Neuroradiol 2005; 26: pp. 1107-1114.
27. Gupta R.K., Nath K., Prasad A., et. al.: In vivo demonstration of neuroinflammatory molecule expression in brain abscess with diffusion tensor imaging. AJNR Am J Neuroradiol 2008; 2: pp. 326-372.
28. Mastroianni C.M., Paoletti F., Lichtner M., et. al.: Cerebrospinal fluid cytokines in patients with tuberculous meningitis. Clin Immunol Immunopathol 1997; 84: pp. 171-176.
29. Sáez-Llorens X., Ramilo O., Mustafa M.M., et. al.: Molecular pathophysiology of bacterial meningitis: current concepts and therapeutic implications. J Pediatr 1990; 116: pp. 671-674.
30. Goldblum S.E., Yoneda K., Cohen P.A., et. al.: Provocation of pulmonary vascular endothelial injury in rabbits by human recombinant interleukin-1β. Infect Immun 1988; 56: pp. 2255-2263.
31. Okusawa S.J., Gelfand A., Ikejima T., et. al.: Interleukin-1 induces a shock-like state in rabbits. J Clin Invest 1988; 81: pp. 1162-1172.
32. Garty B.Z., Berliner S., Liberman E., et. al.: Cerebrospinal fluid leukocyte aggregation in meningitis. Pediatr Infect Dis J 1997; 16: pp. 647-651.
33. Deveci F., Akbulut H.H., Turgut T., et. al.: Changes in serum cytokine levels in active tuberculosis with treatment. Med Inflam 2005; 5: pp. 256-262.
34. Thwaites G.E., Simmons C.P., Quyen N.T.H., et. al.: Pathophysiology and prognosis in Vietnamese adults with tuberculous meningitis. J Infect Dis 2003; 188: pp. 1105-1115.
35. Frick M.A., Wenger D.E., Adkins M.: MR imaging of synovial disorders of the knee: an update. Radiol Clin North Am 2007; 45: pp. 1017-1031.
![Figure 3, Plots (a, b) showing relationship among proinflammatory cytokines (PCs) (tumor necrosis factor-α [TNF-α], soluble intercellular cell adhesion molecules-1 [sICAM-1], and interleukin-1β [IL-1β]), fractional anisotropy (FA) values and postcontrast signal intensity (PCSI) in TBM patients. (c, d) Relationship between FA and PCSI on baseline as well as follow-up study, respectively.](https://storage.googleapis.com/dl.dentistrykey.com/clinical/CorrelationofCSFProinflammatoryCytokineswithMRIinTuberculousMeningitis/2_1s20S1076633209005807.jpg)