Rationale and Objectives
The purpose of this study was to correlate the status of magnetic resonance contrast enhancement with immunohistologic vascular parameters such as microvascular cellular proliferation (MVCP), microvascular density (MVD), vascular endothelial growth factor receptor-2 (VEGFR-2) expression, and World Health Organization (WHO) grade obtained from image-guided biopsy specimens. We also compared perfusion computed tomography (PCT) parameters such as cerebral blood volume (CBV), cerebral blood flow (CBF), and permeability surface area-product (PS) with the presence or absence of contrast enhancement.
Materials and Methods
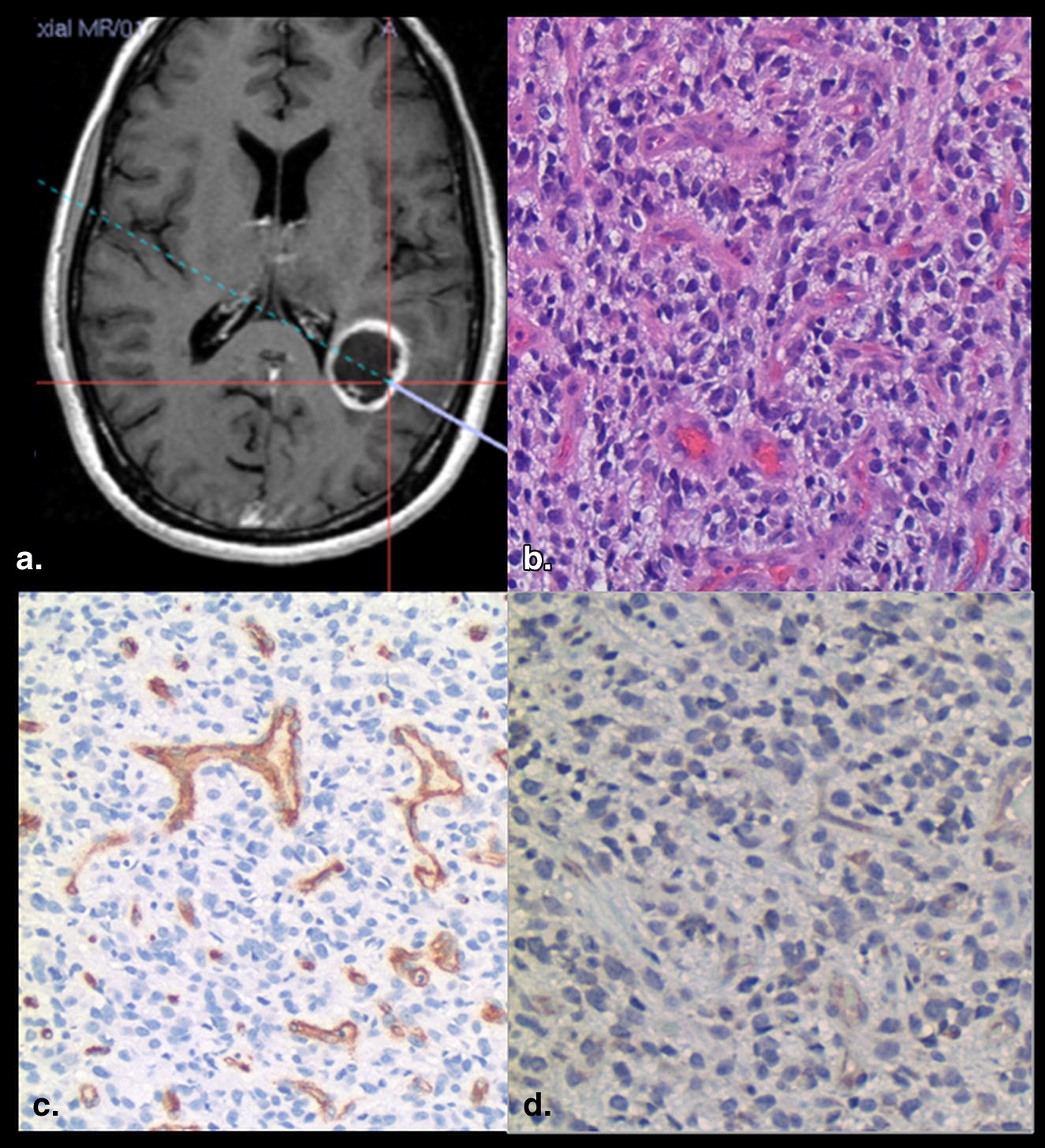
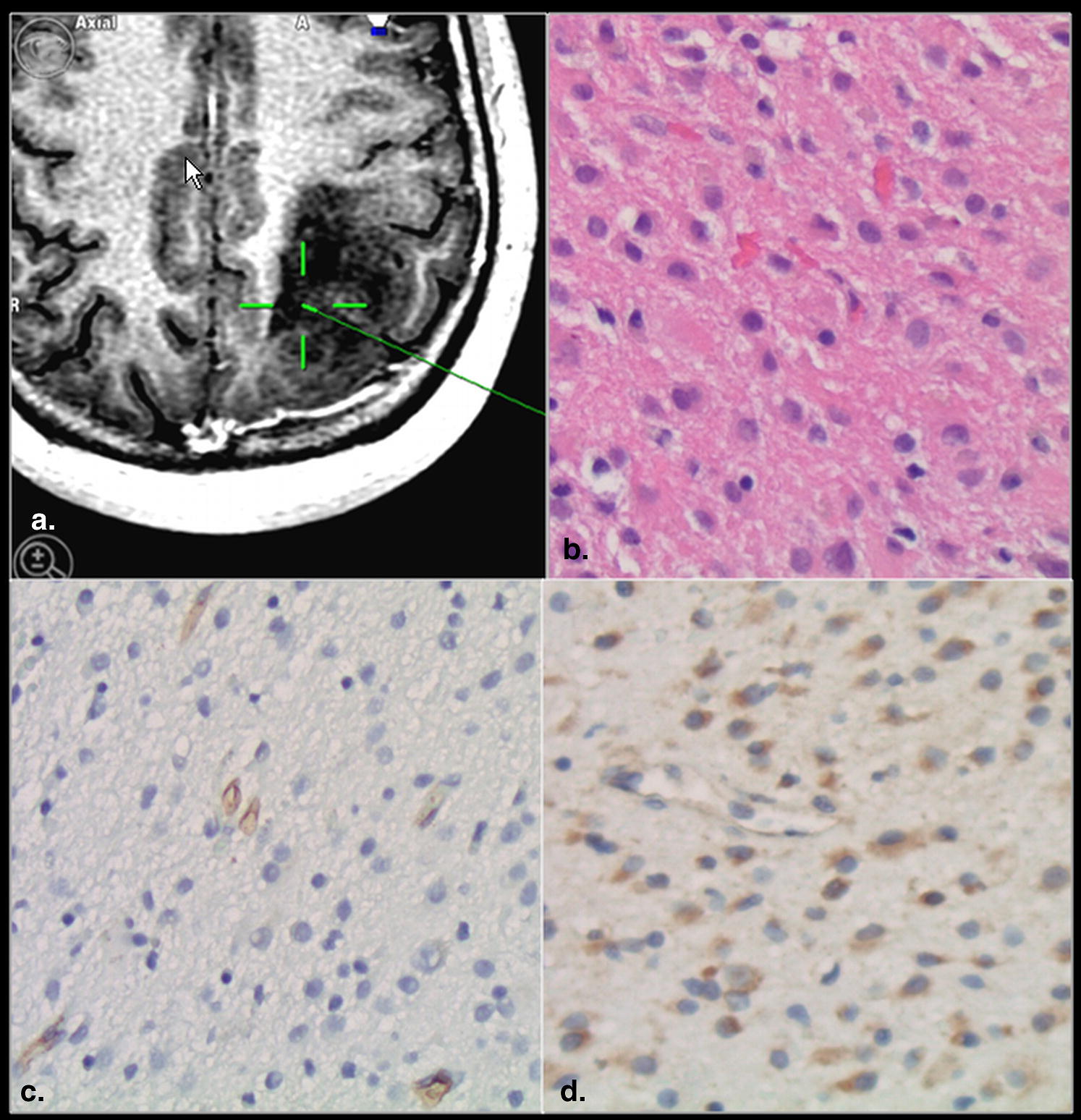
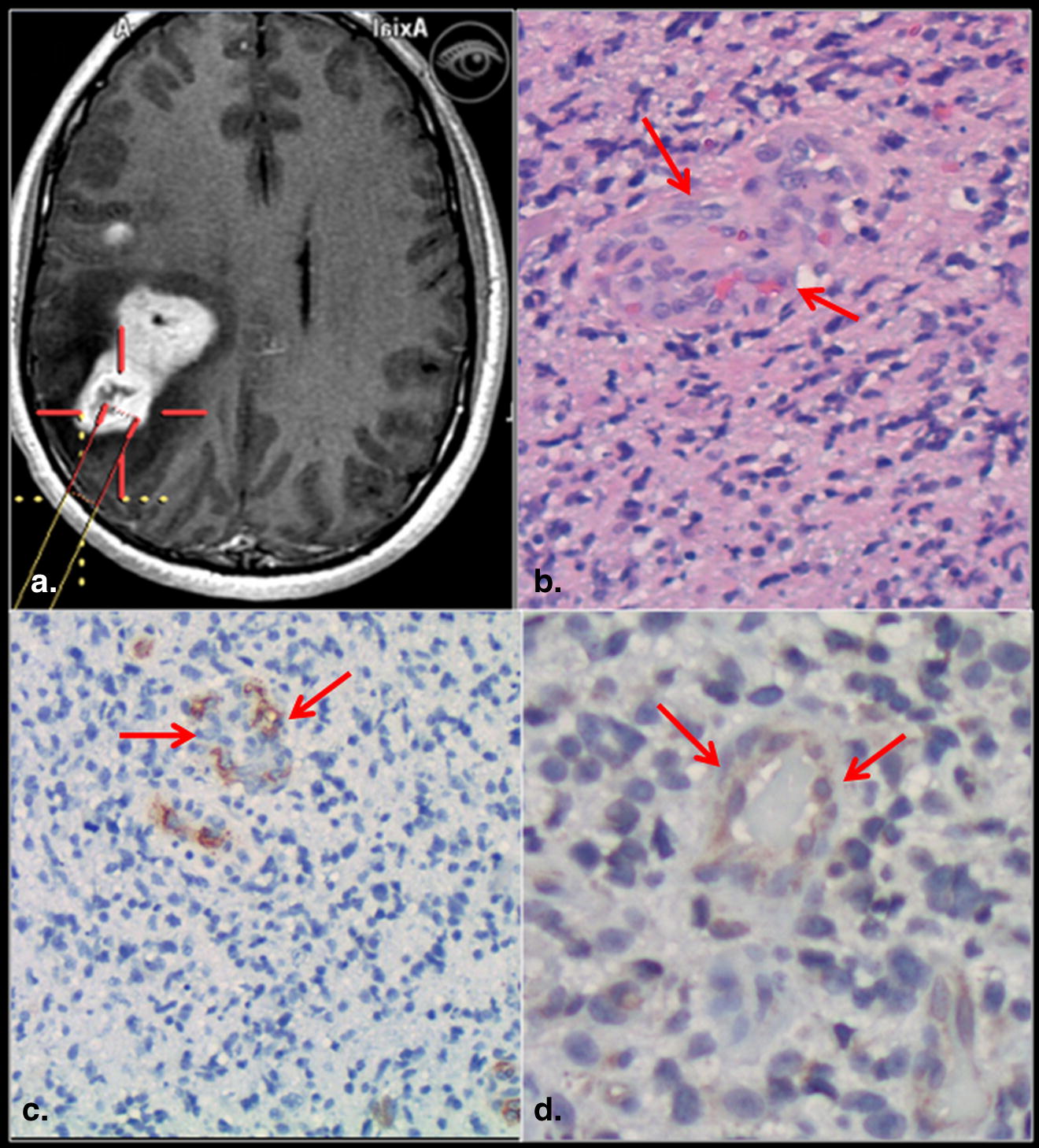
A total of 26 image-guided biopsy specimens in 16 patients with treatment naive gliomas were obtained from contrast-enhancing (CE) and nonenhancing (NE) regions of the glioma. Contrast enhancement status was correlated with MVD, MVCP, VEGFR-2 expression, and WHO grade obtained from the biopsy specimen as well as with the PCT parameters.
Results
Contrast enhancement showed statistically significant correlation with MVCP ( P = .003) and PS ( P = .007) when compared with various immunohistologic and perfusion vascular parameters. WHO grade of the biopsy specimen showed statistically significant correlation with contrast enhancement ( P = .002), MVCP ( P < .001), and PS values ( P = .028).
Conclusion
Contrast enhancement in gliomas is primarily from a break in blood-brain barrier as evidenced by its correlation with PS and MVCP, whereas it was not statistically correlated with CBV and MVD even though it showed a positive trend. Contrast enhancement also showed significant correlation with WHO grade suggesting a biopsy from CE region in a heterogeneous glioma probably will still yield the most aggressive part of the glioma is also shown by its association with MVCP and PS estimates.
Gliomas are usually quite heterogeneous and presence or absence of contrast enhancement may not always indicate the most aggressive part of the tumor. Previous studies have shown that approximately one-third of nonenhancing (NE) gliomas are malignant , whereas 26%–46% of the low-grade tumors may show contrast enhancement . However, despite this predicament, resection or biopsy from the contrast-enhancing (CE) part in enhancing tumors is still the norm, which leads to sampling errors. Recent literature has stressed the role of functional imaging techniques to guide the biopsy to obtain the highest grade of the tumor based on metabolic, physiologic, or hemodynamic status of the tumor ; however, use of the advanced functional imaging techniques is still limited to very few centers.
Tumor angiogenesis is the biologic process by which new capillaries are formed from preexisting vessels and is a critical process for adequate tumor tissue oxygenation and nutritional supply and also tumor invasion and metastasis . Histologic methods to evaluate angiogenesis include both quantitative and qualitative assessment of the tumor vessels, mostly based on microvascular density (MVD), total microvascular area, and microvascular cellular proliferation (MVCP) as well as vascular endothelial growth factor (VEGF) expression. MVD has been used in quantifying tumor angiogenesis and also has been found to be an important independent prognostic indicator for survival in several human cancers . Similarly, MVCP , and VEGF expression have been shown to correlate with tumor grade, aggressiveness, metastatic potential, and hence patient prognosis. These immunohistologic parameters have been shown to correlate with morphologic imaging features such as presence or absence of contrast enhancement as well as with various physiologic measures especially perfusion parameters .
Get Radiology Tree app to read full this article<
Materials and methods
Study Population
Get Radiology Tree app to read full this article<
Image-guided Biopsy
Get Radiology Tree app to read full this article<
Neuropathologic Analysis
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Perfusion CT Technique
Get Radiology Tree app to read full this article<
PCT Map Analysis
Get Radiology Tree app to read full this article<
Statistical Analysis
Get Radiology Tree app to read full this article<
Results
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
CE versus VEGFR-2 Immunoreactivity
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
CE versus MVCP
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
CE versus MVD
Get Radiology Tree app to read full this article<
Contrast Enhancement versus PCT Parameters
Get Radiology Tree app to read full this article<
Table 1
Associations with Contrast Enhancement
Parameter CE ( n = 16) NE ( n = 10)P Value VEGFR-2, n (%) 12/16 (75%) 5/9 (55%) ∗ .164 MVCP, n (%) 9 (56%) 0 (0%) .003 MVD (vessel number) mean (SE) 138.1 (40.1) 87.7 (10.4) .550 CBV (mL/100 g) mean (SE) 2.53 (0.94) 1.25 (0.14) .157 CBF (mL/100 g/min) mean (SE) 69.8 (35.7) 33.0 (5.7) .497 MTT (seconds) mean (SE) 4.49 (0.79) 3.46 (0.68) .388 PS (mL/100 g/min) mean (SE) 3.13 (1.17) 0.44 (0.07) .007
CBF, cerebral blood flow; CBV, cerebral blood volume; CE, contrast enhancing; MTT, mean transit time; MVD, microvascular density; NE, nonenhancing; PS, permeability surface area-product; VEGFR-2, vascular endothelial growth factor receptor-2.
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Associations with Biopsy Grade
Get Radiology Tree app to read full this article<
Table 2
Associations with Biopsy Grade
Parameter Low Grade ( n = 15) High Grade ( n = 10)P Value Contrast enhancement, n (%) 6 (40%) 10 (100%) .002 VEGFR-2, n (%) 8 (53%) 8 (80%) .217 MVCP, n (%) 1 (7%) 8 (80%) <.001 MVD (vessel number) mean (SE) 77.9 (8.8) 183.9 (56.2) .171 CBV (mL/100 g) mean (SE) 1.34 (0.14) 3.20 (1.37) .131 CBF (mL/100 g/min) mean (SE) 29.0 (4.8) 98.5 (51.0) .208 MTT (seconds) mean (SE) 4.39 (0.72) 3.81 (1.08) .509 PS (mL/100 g/min) mean (SE) 0.72 (0.18) 4.31 (1.57) .028
CBF, cerebral blood flow; CBV, cerebral blood volume; CE, contrast enhancing; MTT, mean transit time; MVD, microvascular density; NE, non-enhancing; PS, permeability surface area-product; VEGFR-2, vascular endothelial growth factor receptor-2.
Get Radiology Tree app to read full this article<
WHO Grading of the Biopsy Specimen and Comparison with the Final Grade of the Tumor
Get Radiology Tree app to read full this article<
Discussion
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Limitations of the Study
Get Radiology Tree app to read full this article<
Conclusions
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
References
1. Scott J.N., Brasher P.M., Sevick R.J., et. al.: How often are nonenhancing supratentorial gliomas malignant? A population study. Neurology 2002; 59: pp. 947-949.
2. Barker F.G., Chang S.M., Huhn S.L., et. al.: Age and the risk of anaplasia in magnetic resonance-nonenhancing supratentorial cerebral tumors. Cancer 1997; 80: pp. 936-941.
3. Chaichana K.L., McGirt M.J., Niranjan A., et. al.: Prognostic significance of contrast-enhancing low-grade gliomas in adults and a review of the literature. Neurol Res 2009; 31: pp. 931-939.
4. Dhermain F., Saliou G., Parker F., et. al.: Microvascular leakage and contrast enhancement as prognostic factors for recurrence in unfavorable low-grade gliomas. J Neurooncol 2010; 97: pp. 81-88.
5. Sugahara T., Korogi Y., Kochi M., et. al.: Correlation of MR imaging-determined cerebral blood volume maps with histologic and angiographic determination of vascularity of gliomas. AJR Am J Roentgenol 1998; 171: pp. 1479-1486.
6. Aronen H.J., Gazit I.E., Louis D.N., et. al.: Cerebral blood volume maps of gliomas: comparison with tumor grade and histologic findings. Radiology 1994; 191: pp. 41-51.
7. Provenzale J.M., Wang G.R., Brenner T., et. al.: Comparison of permeability in high-grade and low-grade brain tumors using dynamic susceptibility contrast MR imaging. AJR Am J Roentgenol 2002; 178: pp. 711-716.
8. Law M., Yang S., Wang H., et. al.: Glioma grading: sensitivity, specificity, and predictive values of perfusion MR imaging and proton MR spectroscopic imaging compared with conventional MR imaging. AJNR Am J Neuroradiol 2003; 24: pp. 1989-1998.
9. Folkman J.: The role of angiogenesis in tumor growth. Semin Cancer Biol 1992; 3: pp. 65-71.
10. Weidner N., Semple J.P., Welch W.R., et. al.: Tumor angiogenesis and metastasis—correlation in invasive breast carcinoma. N Engl J Med 1991; 324: pp. 1-8.
11. Weidner N.: Tumoural vascularity as a prognostic factor in cancer patients: the evidence continues to grow. J Pathol 1998; 184: pp. 119-122.
12. Fox S.B.: Tumour angiogenesis and prognosis. Histopathology 1997; 30: pp. 294-301.
13. Tanaka F., Oyanagi H., Takenaka K., et. al.: Glomeruloid microvascular proliferation is superior to intratumoral microvessel density as a prognostic marker in non-small cell lung cancer. Cancer Res 2003; 63: pp. 6791-6794.
14. Wesseling P., Vandersteenhoven J.J., Downey B.T., et. al.: Cellular components of microvascular proliferation in human glial and metastatic brain neoplasms. A light microscopic and immunohistochemical study of formalin-fixed, routinely processed material. Acta Neuropathol 1993; 85: pp. 508-514.
15. Chaudhry I.H., O’Donovan D.G., Brenchley P.E., et. al.: Vascular endothelial growth factor expression correlates with tumour grade and vascularity in gliomas. Histopathology 2001; 39: pp. 409-415.
16. Brat D.J., Van Meir E.G.: Glomeruloid microvascular proliferation orchestrated by VPF/VEGF: A new world of angiogenesis research. Am J Pathol 2001; 158: pp. 789-796.
17. Turetschek K., Preda A., Floyd E., et. al.: MRI monitoring of tumor response following angiogenesis inhibition in an experimental human breast cancer model. Eur J Nucl Med Mol Imaging 2003; 30: pp. 448-455.
18. Maia A.C., Malheiros S.M., da Rocha A.J., et. al.: MR cerebral blood volume maps correlated with vascular endothelial growth factor expression and tumor grade in nonenhancing gliomas. AJNR Am J Neuroradiol 2005; 26: pp. 777-783.
19. Callot V., Galanaud D., Figarella-Branger D., et. al.: Correlations between MR and endothelial hyperplasia in low-grade gliomas. J Magn Reson Imaging 2007; 26: pp. 52-60.
20. Jain R., Gutierrez J., Narang J., et. al.: In vivo correlation of tumor blood volume and permeability with histologic and molecular angiogenic markers in gliomas. AJNR Am J Neuroradiol 2010; Nov 24. [Epub ahead of print]
21. Weidner N.: Current pathologic methods for measuring intratumoral microvessel density within breast carcinoma and other solid tumors. Breast Cancer Res Treat 1995; 36: pp. 169-180.
22. Ginsberg L.E., Fuller G.N., Hashmi M., et. al.: The significance of lack of MR contrast enhancement of supratentorial brain tumors in adults: histopathological evaluation of a series. Surg Neurol 1998; 49: pp. 436-440.
23. Ellika S.K., Jain R., Patel S.C., et. al.: Role of perfusion CT in glioma grading and comparison with conventional MR imaging features. AJNR Am J Neuroradiol 2007; 28: pp. 1981-1987.
24. Jain R., Ellika S.K., Scarpace L., et. al.: Quantitative estimation of permeability surface-area product in astroglial brain tumors using perfusion CT and correlation with histopathologic grade. AJNR Am J Neuroradiol 2008; 29: pp. 694-700.
25. Lev M.H., Ozsunar Y., Henson J.W., et. al.: Glial tumor grading and outcome prediction using dynamic spin-echo MR susceptibility mapping compared with conventional contrast-enhanced MR: confounding effect of elevated rCBV of oligodendrogliomas. AJNR Am J Neuroradiol 2004; 25: pp. 214-221.
26. Weidner N.: Intratumor microvessel density as a prognostic factor in cancer. Am J Pathol 1995; 147: pp. 9-19.
27. Wesseling P., Vandersteenhoven J.J., Downey B.T., et. al.: Cellular components of microvascular proliferation in human glial and metastatic brain neoplasms. A light microscopic and immunohistochemical study of formalin-fixed, routinely processed material. Acta Neuropathol 1993; 85: pp. 508-514.
28. Dvorak H.F., Brown L.F., Detmar M., et. al.: Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am J Pathol 1995; 146: pp. 1029-1039.
29. Tynninen O., Aronen H.J., Ruhala M., et. al.: MRI enhancement and microvascular density in gliomas. Correlation with tumor cell proliferation. Invest Radiol 1999; 34: pp. 427-434.
30. Nishikawa R., Cheng S.Y., Nagashima R., et. al.: Expression of vascular endothelial growth factor in human brain tumors. Acta Neuropathol 1998; 96: pp. 453-462.
31. Plate K.H., Breier G., Weich H.A., et. al.: Vascular endothelial growth factor and glioma angiogenesis: coordinate induction of VEGF receptors, distribution of VEGF protein and possible in vivo regulatory mechanisms. Int J Cancer 1994; 59: pp. 520-529.
32. Samoto K., Ikezaki K., Ono M., et. al.: Expression of vascular endothelial growth factor and its possible relation with neovascularization in human brain tumors. Cancer Res 1995; 55: pp. 1189-1193.
33. Takano S., Yoshii Y., Kondo S., et. al.: Concentration of vascular endothelial growth factor in the serum and tumor tissue of brain tumor patients. Cancer Res 1996; 56: pp. 2185-2190.