Rationale and Objectives
A method of estimating and mapping the cortical damage resulting from neurodegenerative diseases based on diffusion-weighted imaging was recently proposed. We improved on this method to visualize the cortical damage in Alzheimer’s disease (AD) in the lateral and medial aspects of the cerebral hemispheres and to provide anatomic references.
Materials and Methods
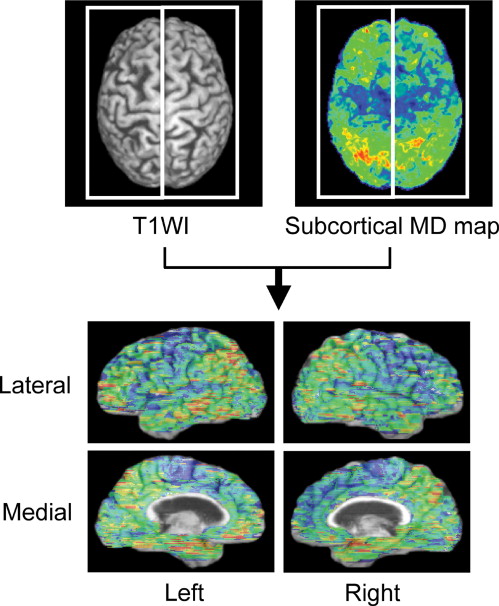
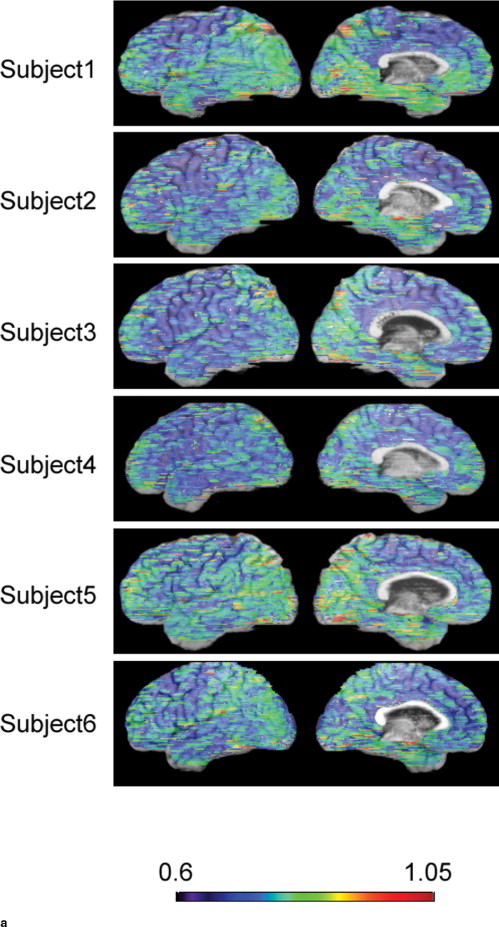
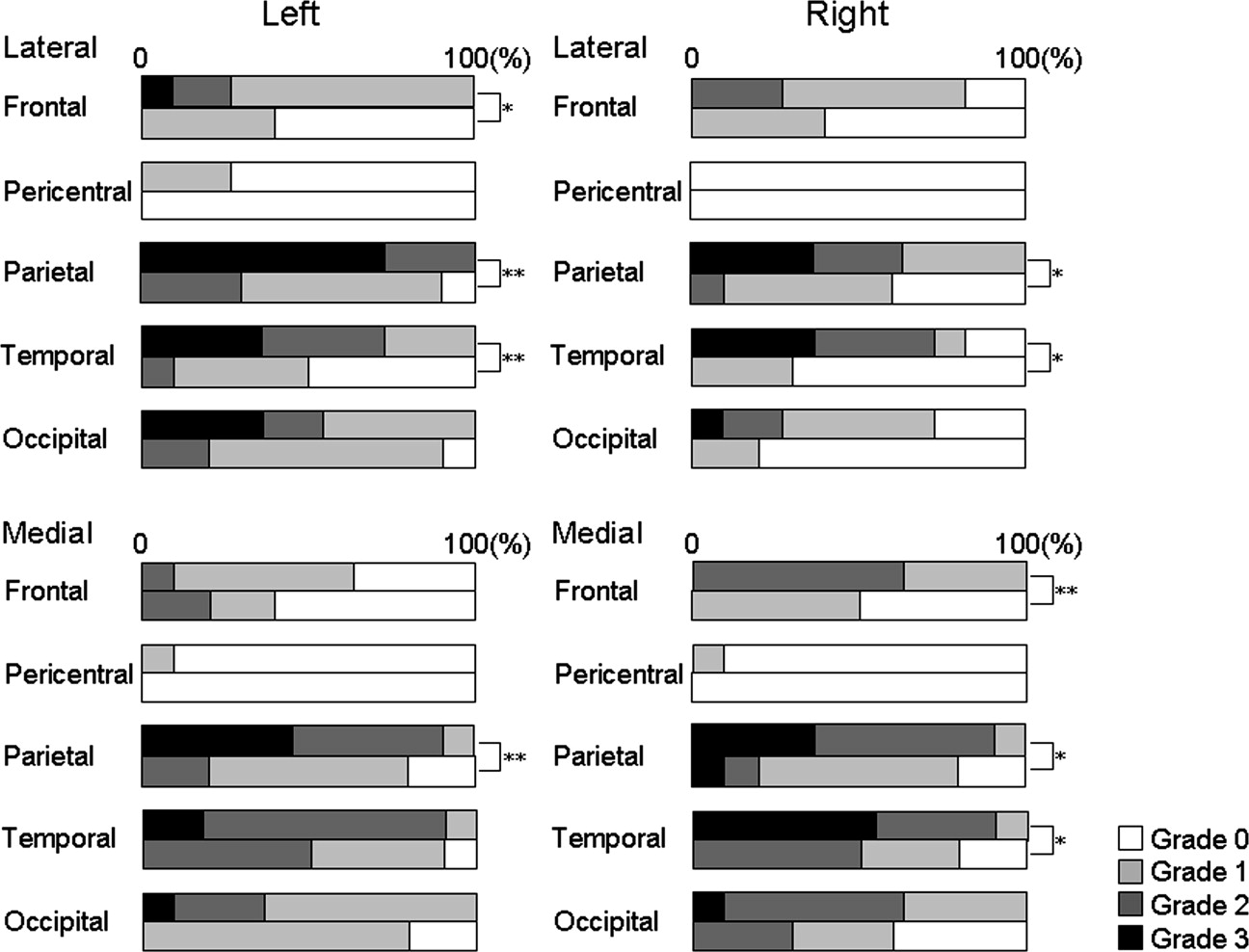
Damage in the cerebral cortex was estimated based on diffusivity in the subcortical white matter according to a previously published method. A map of subcortical mean diffusivity (MD) was superimposed on the corresponding anatomic image so that the spatial extent of the abnormality could be anatomically localized. The right and left hemispheres were separated to evaluate the medial and lateral aspects of each hemisphere. This method was applied to 10 healthy subjects and 11 AD patients. MDs within 20 cortical regions were visually evaluated and statistically compared between AD and healthy subjects at a significance level of P < .01.
Results
In addition to the involvement of the lateral aspects of the bilateral parietal and temporal lobes and clear sparing of the bilateral pericentral regions that were previously reported, significant MD elevation was observed in the medial aspects of the right frontal, bilateral parietal, and right temporal lobes. The extent of MD abnormalities was easily identified by the background anatomic image.
Conclusions
Results suggested that AD damage in the lateral and medial cerebral cortex can be visualized with an anatomic reference using our method.
Diffusion-weighted (DW) imaging has been used to detect the microstructural alteration of brain tissue in neurodegenerative diseases such as Alzheimer’s disease (AD). An abnormally increased apparent diffusion coefficient (ADC) of water molecules in the hippocampus ( ) and an increased ADC and decreased diffusion anisotropy in the white matter regions ( ) have been reported. Although there have been only a few studies of histologic correlation, these abnormalities in DW images in neurodegenerative diseases have been considered to reflect degenerative changes in local brain tissue including neuronal loss, axonal degeneration, demyelination, and gliosis ( ), which result in reduced restriction on the motion of water molecules. In neurodegenerative diseases such as AD, neurons in the gray matter are considered to be the primary site of damage. Evaluation of the cerebral cortex using DW imaging may be of value to investigate the topographic distribution of degenerative damage within the cerebrum. However, direct measurement of the cortical ADC is extremely difficult because of the partial volume averaging of the signal from the adjacent cerebrospinal fluid (CSF), which may result in a substantial overestimation of the ADC. Recently, a DW imaging-based method for estimating the distribution of neurodegenerative damage in the cerebral cortex was proposed ( ). In this method, diffusivity in the subcortical white matter is measured and mapped onto the overlying cortical surface. Preliminary results have shown that the topographic pattern of the subcortical abnormality revealed by this method was in close agreement with that of pathologic cortical abnormality typically observed in patients with AD ( ). Such a method would potentially be useful for assessing the extent of cortical damage, and perhaps could facilitate the differential diagnosis of neurodegenerative diseases.
In this article, we improved on the original method to examine the medial surface of the cerebral hemisphere and to provide an anatomic reference for the estimated cortical damage.
Materials and methods
Subjects
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
MRI Data Acquisition
Get Radiology Tree app to read full this article<
Image Analysis
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Evaluation and Statistical Analysis
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Results
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Discussion
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
References
1. Sandson T.A., Felician O., Edelman R.R., et. al.: Diffusion-weighted magnetic resonance imaging in Alzheimer’s disease. Dement Geriatr Cogn Disord 1999; 10: pp. 166-171.
2. Kantarci K., Jack C.R., Xu Y.C., et. al.: Mild cognitive impairment and Alzheimer’s disease: regional diffusivity of water. Radiology 2001; 219: pp. 101-107.
3. Hanyu H., Shindo H., Kakizaki D., et. al.: Increased water diffusion in cerebral white matter in Alzheimer’s disease. Gerontology 1997; 43: pp. 343-351.
4. Bozzali M., Falini A., Franceschi M., et. al.: White matter damage in Alzheimer’s disease assessed in vivo using diffusion tensor magnetic resonance imaging. J Neurol Neurosurg Psychiatry 2002; 72: pp. 742-746.
5. Yoshiura T., Mihara F., Ogomori K., et. al.: Diffusion tensor in posterior cingulated gyrus: correlation with cognitive decline in Alzheimer’s disease. Neuroreport 2002; 13: pp. 2299-2302.
6. Larsson E.M., Englund E., Sjobeck M., et. al.: MRI with diffusion tensor imaging post-mortem at 3.0 T in a patient with frontotemporal dementia. Dement Geriatr Cogn Disord 2004; 17: pp. 316-319.
7. Yoshiura T., Mihara F., Tanaka A., et. al.: Novel method to estimate and display cerebral cortical degeneration using diffusion-weighted magnetic resonance imaging. Magn Reson Med 2005; 54: pp. 455-459.
8. Yoshiura T., Mihara F., Koga H., et. al.: Mapping of subcortical white matter degeneration using diffusion-weighted magnetic resonance imaging. Acad Radiol 2006; 13: pp. 1460-1464.
9. Heid O. Eddy current-nulled diffusion weighting. In: Proceedings of the 8th Annual Meeting ISMRM, Denver, 2000, p. 799.
10. Kwon K.K., McKinstry R.C., Chien D., et. al.: CSF-suppressed quantitative single-shot diffusion imaging. Magn Reson Med 1991; 21: pp. 157-163.
11. Basser P.J., Pierpaoli C.: Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B 1996; 111: pp. 209-219.
12. Pierpaoli C., Jezzard P., Basser P.J., et. al.: Diffusion tensor MR imaging of the human brain. Radiology 1996; 201: pp. 637-648.
13. Brun A., Gsutafson L.: Distribution of cerebral degeneration in Alzheimer’s disease. Arch Psychiat Nervenkr 1976; 223: pp. 15-33.
14. Braak H., Braak E.: Neuropathological staging of Alzheimer-related changes. Acta Neuropathol 1991; 82: pp. 239-259.
15. O’Sullivan M., Jones D.K., Summers P.E., et. al.: Evidence of cortical ”disconnection” as a mechanism of age-related cognitive decline. Neurology 2001; 57: pp. 632-638.
16. O’Sullivan M., Summers P.E., Jones D.K., et. al.: Normal-appearing white matter in ischemic leukoaraiosis: a diffusion tensor MRI study. Neurology 2001; 57: pp. 2307-2310.