Rationale and Objectives
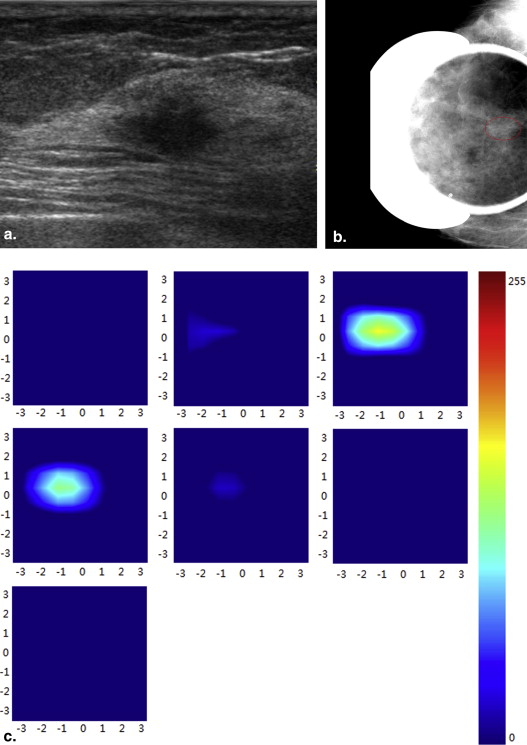
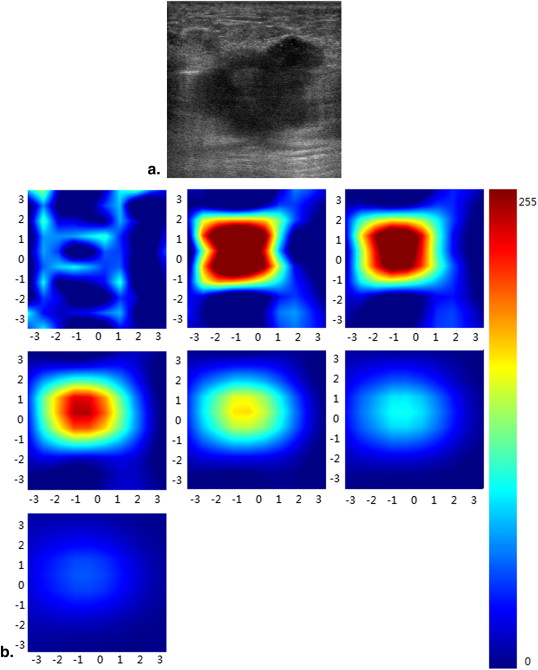
Diffuse optical tomography (DOT) is an emerging functional modality, which can reflect tumor metabolic activity and angiogenesis. The purpose of this exploratory study was to correlate the total hemoglobin concentration (THC) measured by noninvasive DOT with prognostic factors in breast carcinomas.
Materials and Methods
We prospectively imaged 251 breast carcinomas in 229 consecutive women (mean age, 51.18 ± 12.32 years) using DOT from 2007 to 2010. Tumor angiogenesis and metabolic activity were assessed based on quantitatively measured THC. The THC was correlated with prognostic factors, including tumor size, histopathologic classification, histologic grade, estrogen receptor (ER), progesterone receptor (PR), c-erbB-2, and p53.
Results
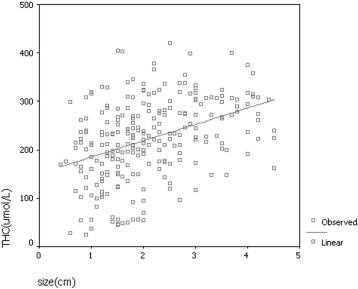
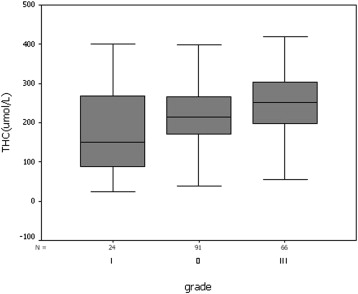
In univariate analysis, THC was significantly correlated with the following prognostic factors: tumor size ( P < .001), histologic grade ( P < .001), ER ( P < .05), PR ( P < .001), and c-erbB-2 ( P < .05). THC was not associated with histopathologic classification ( P = .170) or p53 ( P = .463). On the basis of a stepwise multiple regression analysis, THC of invasive ductal carcinoma was significantly correlated with tumor size ( P < .001), histologic grade ( P < .001), and PR ( P < .05).
Conclusions
THC was associated with prognostic factors of breast carcinoma. THC may be considered as a new prognostic parameter of breast carcinoma and a prediction of tumor behavior and biological activity.
Ultrasound has been widely used as a screening and diagnostic modality to assess the morphologic characteristics of breast tumors. However, they do not provide information on physiological changes in lesions. Diffuse optical tomography (DOT) is an emerging functional modality, which is used for scanning multiwavelength diffuse scattering photons to acquire information on physiology, biochemistry, and molecular function of breast tumors and give three-dimensional maps of absorption. DOT can measure the total hemoglobin concentration (THC) of breast lesions to quantitatively reflect tumor metabolic activity and angiogenesis, which are associated with prognosis in breast carcinoma. Some researchers have used THC to differentiate breast carcinomas from benign lesions and to monitor tumor changes during neoadjuvant chemotherapy . However, to our knowledge, there are few studies that have addressed the associations between THC and histopathologic prognostic factors of breast carcinoma . The purpose of our study was to investigate the correlation between THC and prognostic factors of breast carcinoma and the potential role of THC in predicting biological behavior preoperatively.
Materials and Methods
Patients
The authors prospectively evaluated 546 lesions using ultrasound-guided DOT in 489 consecutive women who underwent open biopsy in our hospital between October 2007 and February 2010. The lesions were all identified using ultrasound at the time of the study. A total of 254 patients with 276 lesions were pathologically diagnosed with breast carcinomas. Eleven patients were excluded because their breast tissue was too thin to image (<1 cm), and 14 carcinomas were excluded because they had a large diameter (>5 cm), which caused poor probe-tissue contact and resulted in image artifacts. Thus, a total of 229 consecutive women (mean age, 51.18 ± 12.32 years; range, 19–82 years) with 251 breast carcinomas were included in the final study. The institutional review board approved this study, and all patients signed informed consent forms.
Ultrasound-guided DOT Imaging Methods
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Histopathologic Analysis
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Immunohistochemical Analysis
Get Radiology Tree app to read full this article<
Statistical Analysis
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Results
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Table 1
Associations Between the THC and Prognostic Factors in Breast Carcinoma
Prognostic factors Number THC (μmol/L)P Value Histopathologic classification Ductal carcinoma in situ 36 245 ± 79 .292 Invasive ductal carcinoma 181 221 ± 83 Invasive lobular carcinoma 16 222 ± 86 Tumor size ≤1 cm 33 180 ± 83 <.001 1.1–2.0 cm 109 199 ± 82 >2 cm 109 258 ± 67 Histological grade Grade I 24 176 ± 108 <.001 Grade II 91 213 ± 73 Grade III 66 249 ± 77 ER Positive 158 211 ± 81 <.05 Negative 73 234 ± 68 PR Positive 160 204 ± 81 <.001 Negative 71 249 ± 60 c-erbB-2 0–2+ 174 213 ± 82 <.05 3+ 55 237 ± 56 p53 Negative 171 216 ± 79 .468 Positive 53 225 ± 73
ER, estrogen receptor; PR, progesterone receptor; THC, total hemoglobin concentration.
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Discussion
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Conclusions
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
References
1. You S.S., Jiang Y.X., Zhu Q.L., et. al.: US-guided diffused optical tomography: a promising functional imaging technique in breast lesions. Eur Radiol 2010; 20: pp. 309-317.
2. Zhu Q., Hegde P.U., Ricci A., et. al.: Early-stage invasive breast cancers: potential role of optical tomography with US localization in assisting diagnosis. Radiology 2010; 256: pp. 367-378.
3. Zhu Q., Tannenbaum S., Hegde P., et. al.: Noninvasive monitoring of breast cancer during neoadjuvant chemotherapy using optical tomography with ultrasound localization. Neoplasia 2008; 10: pp. 1028-1040.
4. Choi J.S., Kim M.J., Youk J.H., et. al.: US-guided optical tomography: correlation with clinicopathologic variables in breast cancer. Ultrasound Med Biol 2013; 39: pp. 233-240.
5. Brown J.Q., Wilke L.G., Geradts J., et. al.: Quantitative optical spectroscopy: a robust tool for direct measurement of breast cancer vascular oxygenation and total hemoglobin content in vivo. Cancer Res 2009; 69: pp. 2919-2926.
6. Xu R., Qiang B., Mao J.: Near infrared imaging of tissue heterogeneity: probe design and sensitivity analysis. Conf Proc IEEE Eng Med Biol Soc 2005; 1: pp. 278-281.
7. Elston C.W., Ellis I.O.: Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology 1991; 19: pp. 403-410.
8. Szabó B.K., Aspelin P., Kristoffersen Wiberg M., et. al.: Invasive breast cancer: correlation of dynamic MR features with prognostic factors. Eur Radiol 2003; 13: pp. 2425-2435.
9. Kato T., Kameoka S., Kimura T., et. al.: Angiogenesis as a predictor of long-term survival for 377 Japanese patients with breast cancer. Breast Cancer Res Treat 2001; 70: pp. 65-74.
10. Elston C.W., Ellis I.O., Pinder S.E.: Pathological prognostic factors in breast cancer. Crit Rev Oncol Hematol 1999; 31: pp. 209-223.
11. Yaghan R., Stanton P.D., Robertson K.W., et. al.: Oestrogen receptor status predicts local recurrence following breast conservation surgery for early breast cancer. Eur J Surg Oncol 1998; 24: pp. 424-426.
12. Lee S.H., Cho N., Kim S.J., et. al.: Correlation between high resolution dynamic MR features and prognostic factors in breast cancer. Korean J Radiol 2008; 9: pp. 10-18.
13. Kim S.H., Cha E.S., Kim H.S., et. al.: Diffusion-weighted imaging of breast cancer: correlation of the apparent diffusion coefficient value with prognostic factors. J Magn Reson Imaging 2009; 30: pp. 615-620.
14. Razek A.A., Gaballa G., Denewer A., et. al.: Invasive ductal carcinoma: correlation of apparent diffusion coefficient value with pathological prognostic factors. NMR Biomed 2010; 23: pp. 619-623.
15. Jeh S.K., Kim S.H., Kim H.S., et. al.: Correlation of the apparent diffusion coefficient value and dynamic magnetic resonance imaging findings with prognostic factors in invasive ductal carcinoma. J Magn Reson Imaging 2011; 33: pp. 102-109.
16. Zhu Q., Huang M., Chen N., et. al.: Ultrasound-guided optical tomographic imaging of malignant and benign breast lesions: initial clinical results of 19 cases. Neoplasia 2003; 5: pp. 379-388.
17. Zhu Q., Cronin E.B., Currier A.A., et. al.: Benign versus malignant breast masses: optical differentiation with US-guided optical imaging reconstruction. Radiology 2005; 237: pp. 57-66.
18. Zhu Q., Kurtzma S.H., Hegde P., et. al.: Utilizing optical tomography with ultrasound localization to image heterogeneous hemoglobin distribution in large breast cancers. Neoplasia 2005; 7: pp. 263-270.
19. Srinivasan S., Pogue B.W., Brooksby B., et. al.: Near-infrared characterization of breast tumors in vivo using spectrally-constrained reconstruction. Technol Cancer Res Treat 2005; 4: pp. 513-526.
20. Weidner N., Folkman J., Pozza F., et. al.: Tumor angiogenesis: a new significant and independent prognostic indicator in early-stage breast carcinoma. J Natl Cancer Inst 1992; 84: pp. 1875-1887.
21. Bosari S., Lee A.K., DeLellis R.A., et. al.: Microvessel quantitation and prognosis in invasive breast carcinoma. Hum Pathol 1992; 23: pp. 755-761.
22. Fox S.B.: Tumour angiogenesis and prognosis. Histopathology 1997; 30: pp. 294-301.
23. Weidner N., Semple J.P., Welch W.R., et. al.: Tumor angiogenesis and metastasis—correlation in invasive breast carcinoma. N Engl J Med 1991; 324: pp. 1-8.
24. Gasparini G., Harris A.L.: Clinical importance of the determination of tumor angiogenesis in breast carcinoma: much more than a new prognostic tool. J Clin Oncol 1995; 13: pp. 765-782.
25. Adams J., Carder P.J., Downey S., et. al.: Vascular endothelial growth factor (VEGF) in breast cancer: comparison of plasma, serum, and tissue VEGF and microvessel density and effects of tamoxifen. Cancer Res 2000; 60: pp. 2898-2905.
26. Zhan Q.M.: Molecular oncology.2005.People’s Medical Publishing HouseBeijingpp. 477-478.
27. Olewniczak S., Chosia M., Kwas A., et. al.: Angiogenesis and some prognostic parameters of invasive ductal breast carcinoma in women. Pol J Pathol 2002; 53: pp. 183-188.
28. Slamon D.J., Clark G.M., Wong S.G., et. al.: Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 1987; 235: pp. 177-182.
29. Makkat S., Luypaert R., Stadnik T., et. al.: Deconvolution-based dynamic contrast-enhanced MR imaging of breast tumors: correlation of tumor blood flow with human epidermal growth factor receptor 2 status and clinicopathologic findings—preliminary results. Radiology 2008; 249: pp. 471-482.
30. Menakuru S.R., Brown N.J., Staton C.A., et. al.: Angiogenesis in pre-malignant conditions. Br J Cancer 2008; 99: pp. 1961-1966.
31. Folkman J.: Angiogenesis and breast cancer. J Clin Oncol 1994; 12: pp. 441-443.
32. Weidner N.: Intratumor microvessel density as a prognostic factor in cancer. Am J Pathol 1995; 147: pp. 9-19.
33. Zhu Q., You S., Jiang Y., et. al.: Detecting angiogenesis in breast tumors: comparison of color Doppler flow imaging with ultrasound-guided diffuse optical tomography. Ultrasound Med Biol 2011; 37: pp. 862-869.
34. Kim M.J., Kim J.Y., Youn J.H., et. al.: US-guided diffuse optical tomography for breast lesions: the reliability of clinical experience. Eur Radiol 2011; 21: pp. 1353-1363.
35. Zhu Q.: Optical tomography with ultrasound localization: initial clinical results and technical challenges. Technol Cancer Res Treat 2005; 4: pp. 235-244.