Rationale and Objectives
Diffusion tensor tractography (DTT) for neural fibers of the head-and-neck region at 3T has not been reported. The purpose of this study was to evaluate the feasibility of using DTT for visualizing neural fibers in the head-and-neck region at 3T and to explore the use of this method in patients with head-and-neck mass lesions.
Materials and Methods
Using a 3T scanner, we obtained magnetic resonance images of the head and neck region in 5 healthy volunteers and 5 patients with head and neck mass lesions. All subjects underwent anatomic T1-weighted and diffusion-tensor imaging using a sequence with six motion-probing gradient orientations, a b value of 800 second/mm 2 , and a 128 × 128 pixel matrix. Fiber tracking was with the continuous tracking method. Different postprocessing parameters were investigated to optimize fiber density detection and minimize noise. In five patients with head-and-neck mass lesions, comparison of tractography results and operative findings with regards to mass and nerve relationship was also performed by two observers.
Results
Using the two regions-of-interest method, the greatest fiber density of presumed inferior alveolar nerves was depicted at a maximum angle of 40° and a minimum fiber length of 10 mm. DTT was successfully depicted in all 5 patients. In 4 patients, the relationship between DTT and operative findings was coincided or similar. The interobserver agreement was good.
Conclusions
DTT of the neural fibers in the head and neck region is feasible using a clinical 3T magnetic resonance scanner. Data from a small number of patients with head-and-neck lesions show good agreement between tractography and operative results.
Diffusion tensor magnetic resonance imaging (DT-MRI) and fiber tractography or diffusion tensor tractography (DTT) can be used to visualize the three-dimensional macroscopic fiber tract architecture . These techniques, widely used to demonstrate specific white matter tracts, are useful for evaluating various pathologic conditions in the brain . They have also been applied to visualize neural fibers of the cranial nerves .
Surgery of the head and neck raises the possibility of cranial or cervical nerve damage . Understanding the relationship between the course of nerve fibers and lesions of the head and neck may help to prevent postoperative neurologic complications. To our knowledge, DTT for neural fibers of the head-and-neck region at 3 T has not been reported, probably because of susceptibility or chemical shift artifacts. DTT with a 3-T MRI scanner using a parallel imaging technique and a fat saturation method may allow visualization of the neural fibers in the head and neck. The purpose of this study was to evaluate the feasibility of DTT to depict neural fibers in the head and neck using a 3-T MRI scanner, a parallel imaging technique, and a fat saturation method.
Materials and methods
Study Subjects
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Table 1
Summary of Patient Characteristics and DTT and Operative Findings in 5 Patients Treated by Surgery
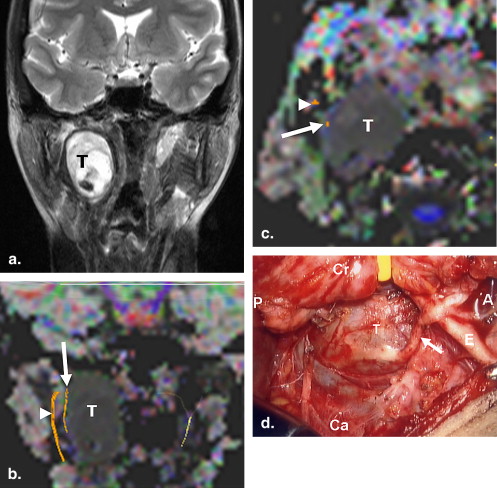
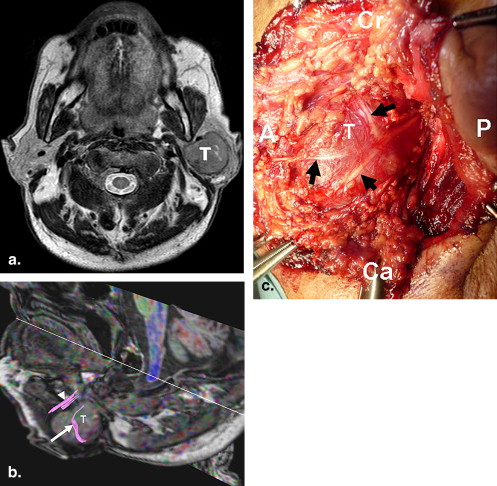
Degree of Coincidence Case/Age/Sex Tumor Type DTT Findings Surgical Findings Observer 1 Observer 2 1/40/F Vagus nerve Schwannoma A tract bundle lateral to a mass A nerve superficial to a mass Grade 2 Grade 2 2/87/F Thyroid carcinoma with LN metastasis Tract bundles lateral to a mass Nerves superficial to a mass Grade 1 Grade 1 3/72/M Warthin tumor Tract bundles lateral and anterior to a mass Nerves superficial to a mass Grade 1 Grade 1 4/66/M Pleomorphic adenoma Tract bundles lateral and medial to a mass Nerves superficial and deep to a mass Grade 1 Grade 1 5/54/M Ductal-type carcinoma Tract bundles lateral to a mass Nerves superficial to a mass Grade 1 Grade 0
DTT: diffusion tensor tractography; LN: lymph node; Grade 2: the relationship of the tracts to the mass depicted on DTT images well coincided with that of the nerves to the mass observed during the operation; Grade 1: the relationship of the tracts to the mass depicted on DTT images partially coincided with that of the nerves to the mass observed during the operation; Grade 0: the relationship of the tracts to the mass depicted on DTT images was not simillar to that of the nerves to the mass observed during the operation.
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
MRI Data Acquisition
Get Radiology Tree app to read full this article<
Image Analysis
Get Radiology Tree app to read full this article<
Fiber Tracking
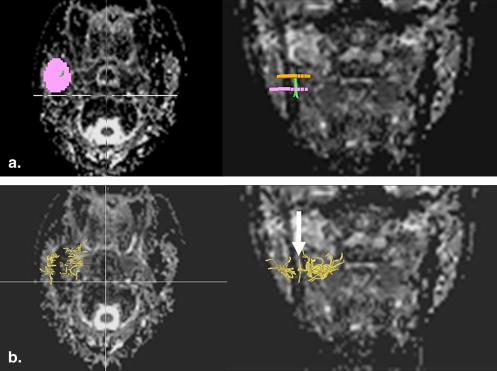
Fiber Tracking optimization
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Technique Exploration in Patients with Head and Neck Mass Lesions
Get Radiology Tree app to read full this article<
Results
Fiber Tracking Optimization
Get Radiology Tree app to read full this article<
Table 2
The Mean Fiber Density of Visualized Tracts in Inferior Alveolar Canal Region on DTT with Single-ROI Method
Maximum Angle 10° 20° 30° 40° 50° 60° 70° Minimum fiber length 10 mm 0.13 ± 0.40 (0/1) 1.05 ± 1.56 (0/7) 2.77 ± 2.43 (10/10) 6.62 ± 4.79 (10/10) 10.4 ± 5.87 (10/10) 16.1 ± 8.70 (10/10) 19.5 ± 9.15 (10/10) 20 mm 0 0.13 ± 0.40 (0/1) 1.08 ± 1.12 (8/8) 3.69 ± 3.15 (10/10) 6.34 ±3.76 (10/10) 8.60 ± 4.08 (10/10) 9.75 ± 5.07 (10/10) 30 mm 0 0 0 0 0.10 ± 0.30 (1/1) 0.61 ± 0.96 (5/5) 1.12 ± 1.82 (5/5)
DTT, diffusion tensor tractography; Minimum fiber length, a minimum length of fiber continuity that is required during fiber tracking; Maximum angle, a maximum angle that prohibited the turning of angles during fiber tracking; ROI, region-of-interest.
The number indicates mean fiber density ± standard deviations. Numbers in parentheses are the number of location having ‘noise’ fibers relative to a total number of location with visualized fibers.
Table 3
The Mean Fiber Density of Visualized Tracts in Inferior Aveolar Canal Region on DTT with Two-ROI Method
Maximum Angle 10° 20° 30° 40° 50° 60° 70° Minimum fiber length 10 mm 0 0.13 ± 0.22 (0/3) 2.01 ± 1.46 (0/9) 6.05 ± 4.03 (0/10) 8.22 ± 3.82 (9/10) 9.43 ± 3.82 (10/10) 10.1 ± 3.87 (10/10) 20 mm 0 0.32 ± 0.10 (0/1) 1.18 ± 1.13 (0/7) 4.24 ± 3.72 (0/10) 5.80 ± 3.83 (8/10) 6.91 ± 3.71 (10/10) 7.55 ± 3.87 (10/10) 30 mm 0 0 0 0 0.03 ± 0.10(1/1) 0.16 ± 0.31(3/3) 0.64 ± 1.29 (4/4)
DTT, diffusion tensor tractography; ROI, region-of-interest.
The number indicates mean fiber density ± standard deviations. Numbers in parantheses are the number of location having “noise” fibers relative to a total number of location with visualized fibers.
Get Radiology Tree app to read full this article<
Technique Exploration in Patients with Head-and-Neck Mass Lesions
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Discussion
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Conclusion
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
References
1. Mori S., Crain B.J., Chacko V.P., et. al.: Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol 1999; 45: pp. 265-269.
2. Basser P.J., Pajevic S., Pierpaoli C., et. al.: In vivo fiber tractography using DT-MRI data. Magn Reson Med 2000; 44: pp. 625-632.
3. Mori S., Van Zijl P.C.: Fiber tracking: principles and strategies—a technical review. NMR Biomed 2002; 15: pp. 468-480.
4. Stieltjes B., Kaufmann W.E., van Zijl P.C., et. al.: Diffusion tensor imaging and axonal tracking in the human brainstem. Neuroimage 2001; 14: pp. 723-735.
5. Dong Q., Welsh R.C., Chenevert T.L., et. al.: Clinical applications of diffusion tensor imaging. J Magn Reson Imaging 2004; 19: pp. 6-18.
6. Wakana S., Jiang H., Nagae-Poetscher L.M., et. al.: Fiber tract-based atlas of human white matter anatomy. Radiology 2004; 230: pp. 77-87.
7. Melhem E.R., Mori S., Mukundan G., et. al.: Diffusion tensor MR imaging of the brain and white matter tractography. AJR Am J Roentgenol 2002; 178: pp. 3-16.
8. Yamada K., Kizu O., Mori S., et. al.: Brain fiber tracking with clinically feasible diffusion-tensor MR imaging: initial experience. Radiology 2003; 227: pp. 295-301.
9. Okada T., Mikuni N., Miki Y., et. al.: Cortispinal tract localization: Integration of diffusion tensor tractography at 3-T MR imaging with intraoperative white matter stimulating mapping—preliminary results. Radiology 2006; 240: pp. 849-857.
10. Huisman T.A., Schwamm L.H., Schaefer P.W., et. al.: Diffusion tensor imaging as potential biomarker of white matter injury in diffuse axonal injury. AJNR Am J Neuroradiol 2004; 25: pp. 370-376.
11. Glenn O.A., Henry R.G., Berman J.I., et. al.: DTI-based three-dimensional tractography detects differences in the pyramidal tracts of infants and children with congenital hemiparesis. J Magn Reson Imaging 2003; 18: pp. 641-648.
12. Lin F., Yu C., Jiang T., et. al.: Diffusion tensor tractography based group mapping of the pyramidal tract in relapsing-remitting multiple sclerosis patients. AJNR Am J Neuroradiol 2007; 28: pp. 278-282.
13. Kunimatsu A., Aoki S., Masutani Y., et. al.: Three-dimensional white matter tractography by diffusion tensor imaging in ischaemic stroke involving the corticospinal tract. Neuroradiology 2003; 45: pp. 532-535.
14. Abe O., Yamada H., Masutani Y., et. al.: Amyotrophic lateral sclerosis: diffusion tensor tractography and voxel-based analysis. NMR Biomed 2004; 17: pp. 411-416.
15. Taoka T., Hirabayashi H., Nakagawa H., et. al.: Displacement of the facial nerve course by vestibular schwannoma: preoperative visualization using diffusion tensor tractography. J Magn Reson Imaging 2006; 24: pp. 1005-1010.
16. Kabasawa H., Masutani Y., Aoki S., et. al.: 3T PROPELLER diffusion tensor fiber tractography: a feasibility study for cranial nerve fiber tracking. Radiat Med 2007; 25: pp. 462-466.
17. Forssell C., Kitzing P., Bergqvist D.: Cranial nerve injuries after carotid artery surgery. A prospective study of 663 operations. Eur J Vasc Endovasc Surg 1995; 10: pp. 445-449.
18. Aimoni C., Lombardi L., Gastaldo E., et. al.: Preoperative and postoperative electroneurographic facial nerve monitoring in patients with parotid tumors. Arch Otolaryngol Head Neck Surg 2003; 129: pp. 940-943.
19. Myssiorek D.: Recurrent laryngeal nerve paralysis: anatomy and etiology. Otolaryngol Clin North Am 2004; 37: pp. 25-44.
20. Kaldoudi E., Williams S.C., Barker G.J., et. al.: A chemical shift selective inversion recovery sequence for fat-suppressed MRI: theory and experimental validation. Magn Reson Imaging 1993; 11: pp. 341-355.
21. asser P.J., Mattiello J., LeBihan D.: MR diffusion tensor spectroscopy and imaging. Biophys J 1994; 66: pp. 259-267.
22. Beaulieu C.: The basis of anisotropic water diffusion in the nervous system: a technical review. NMR Biomed 2002; 15: pp. 435-455.
23. Pierpaoli C., Jezzard P., Basser P.J., et. al.: Diffusion tensor MR imaging of the human brain. Radiology 1996; 201: pp. 637-648.
24. Pierpaoli C., Basser P.J.: Toward a quantitative assessment of diffusion anisotropy. Magn Reson Med 1996; 36: pp. 893-906.
25. Makris N., Worth A.J., Sorensen A.G., et. al.: Morphometry of in vivo human white matter association pathways with diffusion-weighted magnetic resonance imaging. Ann Neurol 1997; 42: pp. 951-962.
26. Pajevic S., Pierpaoli C.: Color schemes to represent the orientation of anisotropic tissues from diffusion tensor data: application to white matter fiber tract mapping in the human brain. Magn Reson Med 1999; 42: pp. 526-540.
27. Fleiss J.L.Statistical methods for rates and proportions.1981.WileyNew York:
28. Okada T., Miki Y., Fushimi Y., et. al.: Diffusion-tensor fiber tractography: intraindividual comparison of 3.0-T and 1.5-T MR imaging. Radiology 2006; 238: pp. 668-678.
29. van den Brink J.S., Watanabe Y., Kuhl C.K., et. al.: Implications of SENSE MR in routine clinical practice. Eur J Radiol 2003; 46: pp. 3-27.
30. Jaermann T., Crelier G., Pruessmann K.P., et. al.: SENSE-DTI at 3 T. Magn Reson Med 2004; 51: pp. 230-236.
31. Kaldoudi E., Williams S.C., Barker G.J., et. al.: A chemical shift selective inversion recovery sequence for fat-suppressed MRI: theory and experimental validation. Magn Reson Imaging 1993; 11: pp. 341-355.
32. Huang H., Zhang J., van Zijl P.C., et. al.: Analysis of noise effects on DTI-based tractography using the brute-force and multi-ROI approach. Magn Reson Med 2004; 52: pp. 559-565.
33. Davis R.A., Anson B.J., Budinger J.M., et. al.: Surgical anatomy of the facial nerve and parotid gland based upon a study of 350 cervicofacial halves. Surg Gynecol Obst 1956; 102: pp. 385-412.
34. Yu C.S., Li K.C., Xuan Y., et. al.: Diffusion tensor tractography in patients with cerebral tumors: a helpful technique for neurosurgical planning and post-operative assessment. Eur J Radiol 2005; 56: pp. 197-204.
35. Frank L.R.: Characterization of anisotropy in high angular resolution diffusion-weighted MRI. Magn Reson Med 2002; 47: pp. 1083-1099.
36. Kreher B.W., Schneider J.F., Mader I., et. al.: Multitensor approach for analysis and tracking of complex fiber configurations. Magn Reson Med 2005; 54: pp. 1216-1225.