Rationale and Objectives
Comparison of two different diffusion weighted (DW) sequences in breast MRI regarding the differentiation between benign and malignant lesions.
Materials and Methods
Breast MRI including two different DW sequences was performed in 165 consecutive women. Inclusion criteria for DW imaging and ADC evaluation were histologically proven focal mass lesions with a diameter of more than 5 mm in dynamic contrast-enhanced MRI. The DW sequences were pre-contrast echo-planar imaging with spectral fat saturation (EPI fs) and DW EPI with inversion recovery (EPI STIR) (b-values: 50, 400, and 800). Lesions were analyzed regarding visibility in DW sequences and ADC values.
Results
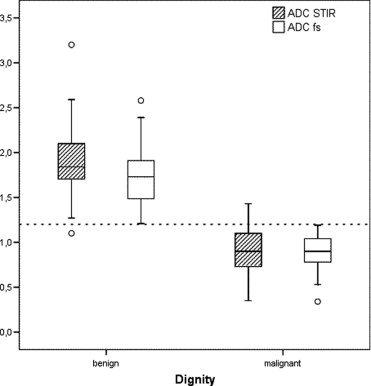
Inclusion criteria were fulfilled in 56 women with 69 lesions. Five lesions could not be evaluated for different reasons. Finally, DW sequences were evaluated in 51 women with 64 focal mass lesions (15 benign, 49 malignant). The visibility of the lesions was significantly better in the EPI fs sequence ( P <0.05). The ADC values (10 −3 mm 2 /s) in the EPI fs were 1.76, 2.58, and 1.21 (mean, maximum, minimum, respectively) for benign lesions and 0.90, 1.19, and 0.34 for malignant lesions. Respective values in the EPI STIR sequence were 1.92, 3.20, 1.10, and 0.91, 1.43, 0.35. Only in the EPI fs sequence there was no overlap in ADC values between benign and malignant lesions.
Conclusion
The DW MRI of the breast with EPI fs and EPI STIR sequences has a high potential to differentiate between benign and malignant breast lesions. Due to better lesion visibility and selectivity, the EPI fs sequence should be preferred.
Breast magnetic resonance imaging (MRI) is an accepted method in detecting primary or recurrent breast cancer in addition to mammography and breast ultrasound. Additionally, it improves preoperative local staging, which is useful for surgical planning ( ). Image analysis is based on the enhancement pattern of lesions in dynamic breast MRI and morphologic changes ( ). With these two criteria, breast MRI has a sensitivity of about 85% to 99% in detecting malignant breast lesions ( ). However, there is an overlap of these criteria with benign lesions, which leads to a reported specificity of about 40% to 80% ( ). In recently published studies, the specificity of breast MRI could be increased using diffusion weighted (DW) sequences ( ). DW MRI is based on the principle that random motion of molecules during the interval of excitation and signal measurement reduces the amplitude of the resulting signal. The application of appropriate pulse sequences (using, e.g., bipolar gradient pulses in one or several directions) allows the measurement of the signal cancellation due to diffusion in the given direction. While normal tissue exhibits gross signal loss, areas with restricted motion of molecules like densely packed tumor cells show less signal loss and become bright in DW images. The value of the diffusion of water in tissue is called the apparent diffusion coefficient (ADC). On basis of the DW images an ADC map can be calculated, which shows the ADC value of each voxel in every slice. Restricted water movement in tumors with high cellularity leads to smaller ADC values ( ). Although this technique is well known in brain imaging, it has not become a routine method in breast MRI. One reason for this may be the high content of fatty tissue in the breast, which makes fat saturation techniques necessary to identify the lesions in the DW images. There are two main possibilities for fat saturation: spectral fat saturation (fs) and a 180° prepulse with a short inversion time (STIR). To our knowledge there is no published study that compares both fat saturation techniques in DW breast MRI in terms of lesion detection and lesion characterization according to the ADC value.
Methods and materials
Patients
From March to November 2006, we performed 233 breast MRI examinations. Patients who took part in another study (n = 54) and patients who refused DW sequences (n = 14) were excluded. All other patients (n = 165) gave informed consent to apply the DW sequences additionally to our normal breast MRI protocol. The study was approved by the local ethics committee.
MRI
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Image Analysis
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Statistical Analysis
Get Radiology Tree app to read full this article<
Results
Get Radiology Tree app to read full this article<
Table 1
Histology of 69 Lesions with DW Sequences
Histology (N = 69) IDC ILC DCIS Rare FA FD BP Total Evaluated 39 6 1 3 8 6 1 64 Not evaluated 2 2 0 1 0 0 0 5
IDC, invasive ductal carcinoma; ILC, invasive lobular carcinoma; DCIS, ductal carcinoma in situ; Rare, rare malignant tumors (medullary, tubular carcinoma, carcinosarcoma, angiosarcoma); FA, fibroadenoma; FD, fibrocystic disease; BP, benign phylloides tumor.
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
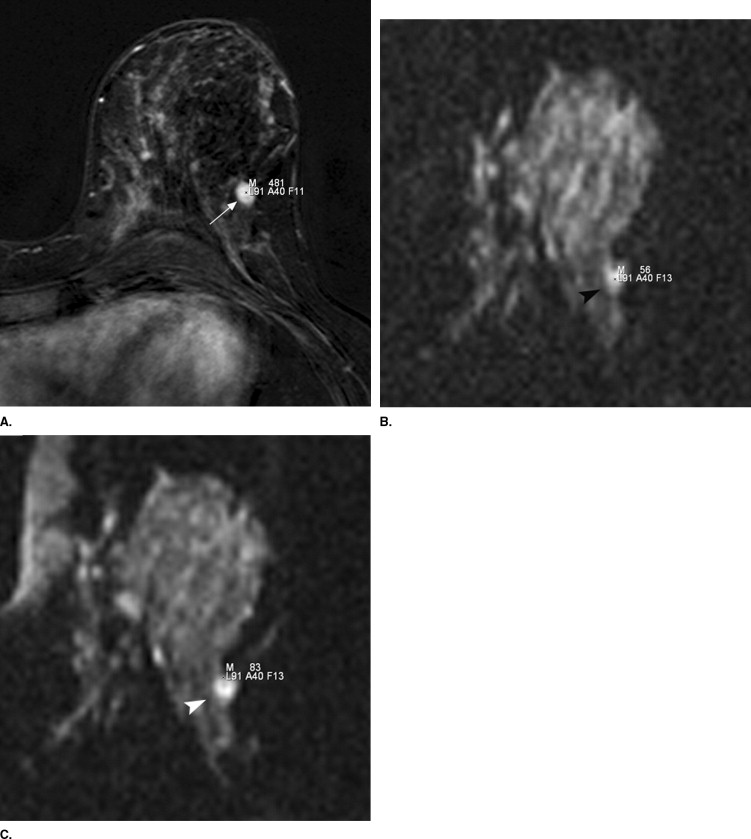
Lesion Delineation in the DW Images
Get Radiology Tree app to read full this article<
Table 2
Lesion Delineation in DW EPI STIR and DW EPI fs
Delineation DW EPI STIR DW EPI fs b m b m 1 6 27 7 38 2 4 21 5 11 3 5 1 3 0 4 0 0 0 0 Σ 15 49 15 49
Delineation of all lesions (n = 64; 15 benign, 49 malignant) in a four point scale (1 = good; 2 = moderate; 3 = poor; 4 = not visible) in both diffusion weighted (DW) pulse sequences.
EPI STIR, echo-planar imaging with short time inversion recovery; EPI fs, echo-planar imaging with spectral fat saturation.
Get Radiology Tree app to read full this article<
ADC Values
Get Radiology Tree app to read full this article<
Table 3
Apparent Diffusion Coefficient (ADC) Values (10 −3 mm 2 /sec) of All Evaluated Lesions (b = benign, m = malignant) in Both MR Diffusion Weighted Sequences
ADC values (10 −3 mm 2 /sec) n Mean ± SD Maximum Minimum 95% CI b m b m b m b m b m DW EPI STIR 15 45 1.92 ± 0.53 0.91 ± 0.24 3.20 1.43 1.10 0.35 1.62–2.22 0.83–0.98 DW EPI fs 15 49 1.76 ± 0.42 0.90 ± 0.18 2.58 1.19 1.21 0.34 1.53–2.00 0.85–0.96
SD, standard deviation; EPI STIR, echo-planar imaging with short time inversion recovery; EPI fs, echo-planar imaging with spectral fat saturation.
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Discussion
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Table 4
Apparent Diffusion Coefficient (ADC) Values (10 −3 mm 2 /sec) of Benign (b) and Malignant Lesions (m) in Three Prior Published Studies
ADC values (10 −3 mm 2 /sec) n Mean ± SD b m b m Rubesova et al. ( ) 22 65 1.51 ± 0.32 0.95 ± 0.22 Kuroki et al. ( ) 5 55 1.48 ± 0.45 1.02 ± 0.23 Woodhams et al. ( ) 24 191 1.67 ± 0.54 1.22 ± 0.31
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Conclusion
Get Radiology Tree app to read full this article<
References
1. Bedrosian I., Mick R., Orel S.G., et. al.: Changes in the surgical management of patients with breast carcinoma based on preoperative magnetic resonance imaging. Cancer 2003; 98: pp. 468-473.
2. Fischer U., Kopka L., Grabbe E.: Breast carcinoma: Effect of preoperative contrast-enhanced MR imaging on the therapeutic approach. Radiology 1999; 213: pp. 881-888.
3. Macura K.J., Ouwerkerk R., Jacobs M.A., et. al.: Patterns of enhancement on breast MR images: Interpretation and imaging pitfalls. Radiographics 2006; 26: pp. 1719-1734. quiz 1719
4. Schnall M.D., Blume J., Bluemke D.A., et. al.: Diagnostic architectural and dynamic features at breast MR imaging: Multicenter study. Radiology 2006; 238: pp. 42-53.
5. Szabo B.K., Aspelin P., Wiberg M.K., et. al.: Dynamic MR imaging of the breast. Acta Radiol 2003; 44: pp. 379-386.
6. Bluemke D.A., Gatsonis C.A., Chen M.H., et. al.: Magnetic resonance imaging of the breast prior to biopsy. JAMA 2004; 292: pp. 2735-2742.
7. Wiener J.I., Schilling K.J., Adami C., et. al.: Assessment of suspected breast cancer by MRI: A prospective clinical trial using a combined kinetic and morphologic analysis. AJR Am J Roentgenol 2005; 184: pp. 878-886.
8. Heywang-Kobrunner S.H., Bick U., Bradley W.G., et. al.: International investigation of breast MRI: Results of a multicentre study (11 sites) concerning diagnostic parameters for contrast-enhanced MRI based on 519 histopathologically correlated lesions. Eur Radiol 2001; 11: pp. 531-546.
9. Kaiser W.A.: (Magnetic resonance tomography of the breast. The results of 253 examinations). Dtsch Med Wochenschr 1989; 114: pp. 1351-1357.
10. Kinkel K., Helbich T.H., Esserman L.J., et. al.: Dynamic high-spatial-resolution MR imaging of suspicious breast lesions: Diagnostic criteria and interobserver variability. AJR Am J Roentgenol 2000; 175: pp. 35-43.
11. Kuhl C.K., Mielcareck P., Klaschik S., et. al.: Dynamic breast MR imaging: Are signal intensity time course data useful for differential diagnosis of enhancing lesions?. Radiology 1999; 211: pp. 101-110.
12. Sinha S., Lucas-Quesada F.A., Sinha U., et. al.: In vivo diffusion-weighted MRI of the breast: Potential for lesion characterization. J Magn Reson Imaging 2002; 15: pp. 693-704.
13. Guo Y., Cai Y.Q., Cai Z.L., et. al.: Differentiation of clinically benign and malignant breast lesions using diffusion-weighted imaging. J Magn Reson Imaging 2002; 16: pp. 172-178.
14. Rubesova E., Grell A.S., De Maertelaer V., et. al.: Quantitative diffusion imaging in breast cancer: A clinical prospective study. J Magn Reson Imaging 2006; 24: pp. 319-324.
15. Woodhams R., Matsunaga K., Kan S., et. al.: ADC mapping of benign and malignant breast tumors. Magn Reson Med Sci 2005; 4: pp. 35-42.
16. Kuroki Y., Nasu K., Kuroki S., et. al.: Diffusion-weighted imaging of breast cancer with the sensitivity encoding technique: Analysis of the apparent diffusion coefficient value. Magn Reson Med Sci 2004; 3: pp. 79-85.
17. Pisano E.D., Gatsonis C., Hendrick E., et. al.: Diagnostic performance of digital versus film mammography for breast-cancer screening. N Engl J Med 2005; 353: pp. 1773-1783.
18. Schulz-Wendtland R., Bock K., Aichinger U., et. al.: (Ultrasound examination of the breast with 7.5 MHz and 13 MHz-transducers: Scope for improving diagnostic accuracy in complementary breast diagnostics?). Ultraschall Med 2005; 26: pp. 209-215.
19. Bartella L., Liberman L., Morris E.A., et. al.: Nonpalpable mammographically occult invasive breast cancers detected by MRI. AJR Am J Roentgenol 2006; 186: pp. 865-870.
20. Kuhl C.K., Klaschik S., Mielcarek P., et. al.: Do T2-weighted pulse sequences help with the differential diagnosis of enhancing lesions in dynamic breast MRI?. J Magn Reson Imaging 1999; 9: pp. 187-196.
21. Nunes L.W., Schnall M.D., Orel S.G.: Update of breast MR imaging architectural interpretation model. Radiology 2001; 219: pp. 484-494.
22. Kuhl C.K., Jost P., Morakkabati N., et. al.: Contrast-enhanced MR imaging of the breast at 3.0 and 1.5 T in the same patients: Initial experience. Radiology 2006; 239: pp. 666-676.
23. Liberman L., Mason G., Morris E.A., et. al.: Does size matter?. AJR Am J Roentgenol 2006; 186: pp. 426-430.
24. Chang S.C., Lai P.H., Chen W.L., et. al.: Diffusion-weighted MRI features of brain abscess and cystic or necrotic brain tumors: Comparison with conventional MRI. Clin Imaging 2002; 26: pp. 227-236.
25. Dorenbeck U., Butz B., Schlaier J., et. al.: Diffusion-weighted echo-planar MRI of the brain with calculated ADCs: A useful tool in the differential diagnosis of tumor necrosis from abscess?. J Neuroimaging 2003; 13: pp. 330-338.
26. Kawashima M., Tamaki Y., Nonaka T., et. al.: MR imaging of mucinous carcinoma of the breast. AJR Am J Roentgenol 2002; 179: pp. 179-183.