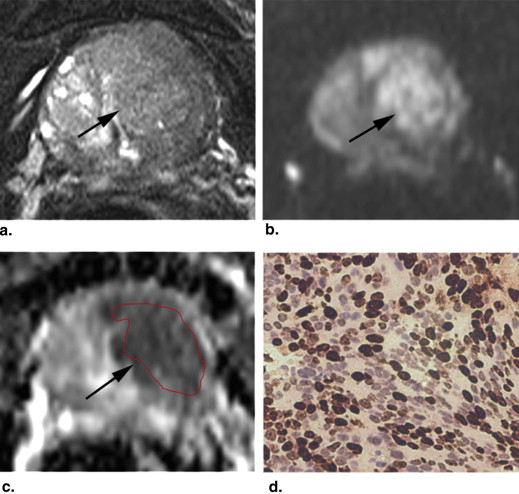
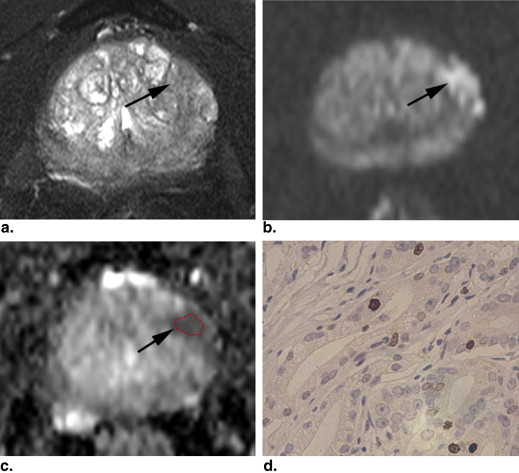
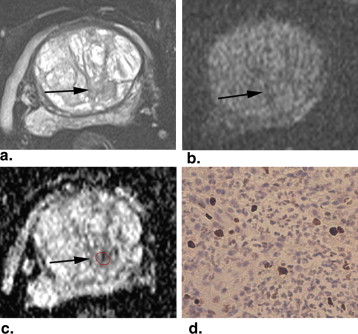
Rationale and Objectives
To investigate the relationship between apparent diffusion coefficient (ADC) values and the Ki-67 staining index (Ki-67 SI), a tumor proliferation marker, in prostate cancer (PCa).
Materials and Methods
Forty-three patients with PCa and thirty-six patients with benign prostatic hyperplasia (BPH) underwent diffusion-weighted (DW) imaging on 3T magnetic resonance (MR) with pelvic phased-array coil. The ADC values of PCa were calculated from two DW images ( b = 0, 800 s/mm 2 ). Immunohistochemical staining for Ki-67 was used to determine the Ki-67 SI of PCa and BPH. The Pearson correlation test was used to examine the relationship between ADC values and the Ki-67 SI. The ADC values of PCa with different level of Ki-67 SI were compared using an independent-sample t -test.
Results
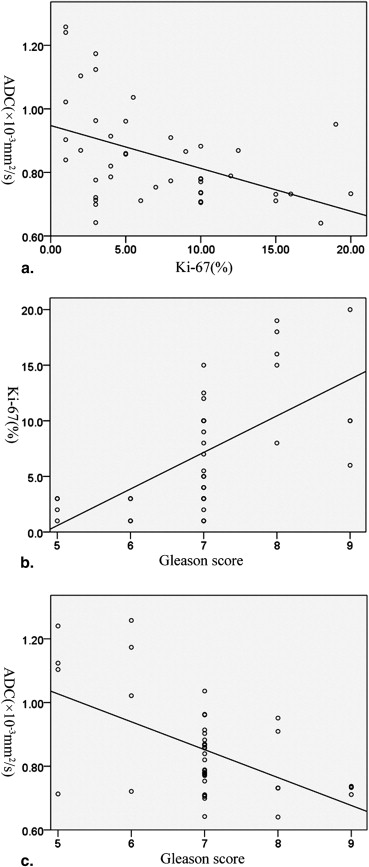
The mean (±standard deviation [SD]) Ki-67 SI of PCa (7.23 ± 5.29%) was higher than that of BPH (2.11 ± 1.90%) ( P < .001). The mean (±SD) ADC value (10 −3 mm 2 /s) of PCa (0.850 ± 0.155) was lower than that of BPH (1.173 ± 0.245) ( P < .001). The ADC values of PCa were negatively correlated with the Ki-67 SI ( r = −0.459, P = .002). The mean ADC values of PCa with Ki-67 >3.5% and ≤3.5% were (0.803 ± 0.094) and (0.936 ± 0.208), respectively. The former was significantly lower than the latter ( P = .031). The ADC values of PCa with Ki-67 >7.1% and ≤7.1% were (0.779 ± 0.081) and (0.906 ± 0.178), respectively. The difference was significant ( P = .004).
Conclusion
The ADC values of PCa could reflect the tumor proliferative activity and the differentiated degree of PCa.
Prostate cancer (PCa) is one of the most common malignant tumors in males in America and Europe. The incidence of PCa in China has increased gradually in recent years. PCa has many available treatment alternatives, yet selecting one optimal option is difficult . Knowledge of the tumor aggressiveness before treatment helps doctors predict the prognosis of patients and personalize treatment. Tumor proliferation activity is one of the indicators to evaluate the aggressiveness. Ki-67 is a proliferation-related nuclear antigen and is expressed in all cycling cells except for resting cells in the G0 phase. The Ki-67 staining index (SI) reflects the proliferation activity of tissue. Furthermore, the Ki-67 SI is a marker of tumor progression, treatment outcome, biochemical recurrence, and progression-free survival . The relationship between Ki-67 SI and clinical results, such as Gleason score, prostate-specific antigen (PSA), and stage, has been investigated in previous studies .
Magnetic resonance imaging (MRI), a noninvasive method, has been used in staging , localizing , and assessing the aggressiveness of PCa . The diffusion-weighted (DW) imaging was one of the most important MRI techniques. It evaluates the random Brownian motion property of water molecules in tissue. The apparent diffusion coefficient (ADC) can be calculated from two or more DW images obtained with different b values and can avoid the T2 shine-through effect on DW images. PCa exhibits impeded diffusion compared with surrounding tissue, manifesting with hyperintense on DW images and hypointense on ADC maps. DW imaging and ADC values have been demonstrated to be useful tools in evaluating the diagnostic accuracy of PCa, which varies in different studies . DW imaging plays an important role in assessing the aggressiveness of PCa by correlating ADC value with Gleason grade . The correlation of ADC values with Ki-67 SI has not been systematically investigated. The purpose of this study was to investigate the relationship between ADC values and Ki-67 SI in PCa.
Materials and methods
Patients
Get Radiology Tree app to read full this article<
MRI Protocols
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Pathological Analysis
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Immunohistochemical Analysis of Ki-67
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
MRI Analysis
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
meanADC=(∑ni=1ADCiSi)/∑ni=1Si mean
ADC
=
(
∑
i
=
1
n
A
D
C
i
S
i
)
/
∑
i
=
1
n
S
i
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Statistical Analysis
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Results
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Table 1
Distribution and ADC Values of Patients with Ki-67 SI by the 3.5% and 7.1% Cutpoint
Ki-67 SI (%) Gleason score ∗ Number of patients ADC value(×10 −3 mm 2 /s)P value 5 6 7 8 9 ≤3.5 4 4 7 0 0 15 (34.9%) 0.936 ± 0.208 .031 >3.5 0 0 19 5 4 28 (65.1%) 0.803 ± 0.094 ≤7.1 4 4 15 0 1 24 (55.8%) 0.906 ± 0.178 .004 >7.1 0 0 11 5 3 19 (44.2%) 0.779 ± 0.081
ADC, apparent diffusion coefficient; SI, staining index.
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Discussion
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Conclusions
Get Radiology Tree app to read full this article<
Acknowledgment
Get Radiology Tree app to read full this article<
References
1. Davison B.J., Breckon E.: Factors influencing treatment decision making and information preferences of prostate cancer patients on active surveillance. Patient Educ Couns 2012; 87: pp. 369-374.
2. Jani A.B., Hellman S.: Early prostate cancer: clinical decision-making. Lancet 2003; 361: pp. 1045-1053.
3. Pollack A., DeSilvio M., Khor L.Y., et. al.: Ki-67 staining is a strong predictor of distant metastasis and mortality for men with prostate cancer treated with radiotherapy plus androgen deprivation: Radiation Therapy Oncology Group Trial 92-02. J Clinl Oncol 2004; 22: pp. 2133-2140.
4. Tran P.T., Hales R.K., Zeng J., et. al.: Tissue biomarkers for prostate cancer radiation therapy. Curr Mol Med 2012; 12: pp. 772-787.
5. Fisher G., Yang Z.H., Kudahetti S., et. al.: Prognostic value of Ki-67 for prostate cancer death in a conservatively managed cohort. Br J Cancer 2013; 108: pp. 271-277.
6. Antonarakis E.S., Keizman D., Zhang Z., et. al.: An immunohistochemical signature comprising PTEN, MYC, and Ki67 predicts progression in prostate cancer patients receiving adjuvant docetaxel after prostatectomy. Cancer 2012; 118: pp. 6063-6071.
7. Khoo V.S., Pollack A., Cowen D., et. al.: Relationship of Ki-67 labeling index to DNA-Ploidy, S-phase fraction, and outcome in prostate cancer treated with radiotherapy. Prostate 1999; 41: pp. 166-172.
8. Luczynska E., Gasinska A., Wilk W.: Expression of Ki-67 (MIB-1) and GLUT-1 proteins in non-advanced prostatic cancer. Pol J Pathol 2012; 63: pp. 272-277.
9. Fütterer J.J.: MR imaging in local staging of prostate cancer. Eur J Radiol 2007; 63: pp. 328-334.
10. Haider M.A., van der Kwast T.H., Tanguay J., et. al.: Combined T2-weighted and diffusion-weighted MRI for localization of prostate cancer. AJR Am J Roentgenol 2007; 189: pp. 323-328.
11. Kobus T., Vos P.C., Hambrock T., et. al.: Prostate cancer aggressiveness: in vivo assessment of MR spectroscopy and diffusion-weighted imaging at 3 T. Radiology 2012; 265: pp. 457-467.
12. Wu L.M., Xu J.R., Gu H.Y., et. al.: Usefulness of diffusion-weighted magnetic resonance imaging in the diagnosis of prostate cancer. Acad Radiol 2012; 19: pp. 1215-1224.
13. Woodfield C.A., Tung G.A., Grand D.J., et. al.: Diffusion-weighted MRI of peripheral zone prostate cancer: comparison of tumor apparent diffusion coefficient with Gleason score and percentage of tumor on core biopsy. AJR Am J Roentgenol 2010; 194: pp. W316-W322.
14. Hambrock T., Somford D.M., Huisman H.J., et. al.: Relationship between apparent diffusion coefficients at 3.0-T MR imaging and Gleason grade in peripheral zone prostate cancer. Radiology 2011; 259: pp. 453-461.
15. Vargas H.A., Akin O., Franiel T., et. al.: Diffusion-weighted endorectal MR imaging at 3T for prostate cancer: tumor detection and assessment of aggressiveness. Radiology 2011; 259: pp. 775-784.
16. Scheenen T.W., Heijmink S.W., Roell S.A., et. al.: Three-dimensional proton MR spectroscopy of human prostate at 3 T without endorectal coil: feasibility. Radiology 2007; 245: pp. 507-516.
17. Li R., Heydon K., Hammond M.E., et. al.: Ki-67 staining index predicts distant metastasis and survival in locally advanced prostate cancer treated with radiotherapy: an analysis of patients in Radiation Therapy Oncology Group Protocol 86-10. Clin Cancer Res 2004; 10: pp. 4118-4124.
18. Wang X.Z., Wang B., Gao Z.Q., et. al.: Diffusion-weighted imaging of prostate cancer: correlation between apparent diffusion coefficient values and tumor proliferation. J Magn Reson Imaging 2009; 29: pp. 1360-1366.
19. Taftachi R., Ayhan A., Ekici S., et. al.: Proliferating-cell nuclear antigen (PCNA) as an independent prognostic marker in patients after prostatectomy: a comparison of PCNA and Ki-67. BJU Int 2005; 95: pp. 650-654.
20. Wang X.Z., Wang B., Gao Z.Q., et. al.: 1H-MRSI of prostate cancer: the relationship between metabolite ratio and tumor proliferation. Eur J Radiol 2010; 73: pp. 345-351.
21. Shukla-Dave A., Hricak H., Ishill N.M., et. al.: Correlation of MR imaging and MR spectroscopic imaging findings with Ki-67, phosphor-Akt, and androgen receptor expression in prostate cancer. Radiology 2009; 250: pp. 803-812.
22. Le Bihan D.: Molecular diffusion, tissue microdynamics and microstructure. NMR Biomed 1995; 8: pp. 375-386.
23. Huang H., Wylie J.J., Miura R.M.: Restricted diffusion in cellular median: (1+1)-dimensional model. Bull Math Biol 2011; 73: pp. 1682-1694.
24. Zhong W., Peng J., He H., et. al.: Ki-67 and PCNA expression in prostate cancer and benign prostatic hyperplasia. Clin Invest Med 2008; 31: pp. E8-E15.
25. Nikoleishvili D., Pertia A., Trsintsadze O., et. al.: Expression of p27((Kip1)), cyclin D3 and Ki67 in BPH, prostate cancer and hormone-treated prostate cancer cells. Int Urol Nephrol 2008; 40: pp. 953-959.
26. Langer D.L., van der Kwanst T.H., Evans A.J., et. al.: Prostate tissue composition and MR measurements: investigating the relationships between ADC, T2, K(trans), v(e), and corresponding histologic features. Radiology 2010; 255: pp. 485-494.
27. Husband J.E., Padhani A.R., MacVicar A.D., et. al.: Magnetic resonance imaging of prostate cancer: comparison of image quality using endorectal and pelvic phased array coils. Clin Radiol 1998; 53: pp. 673-681.
28. Shimizu T., Nishie A., Ro T., et. al.: Prostate cancer detection: the value of performing an MRI before a biopsy. Acta Radiol 2009; 50: pp. 1080-1088.