The aim of this work is to review the techniques and clinical applications of diffusion-weighted magnetic resonance (MR) imaging of the breast. Diffusion-weighted MR imaging plays a role in the differentiation breast cancer from benign lesions, the characterization of malignancy, and the detection of tumor extension. The apparent diffusion coefficient of breast cancer is correlated with tumor cellularity and some prognostic factors of breast cancer. It can be used for the differentiation of recurrent tumors from posttreatment changes and monitoring of patients after chemotherapy. Diffusion-weighted MR imaging is used for the characterization of breast mass, diagnosis, and the grading and staging of breast cancer, as well as prediction of the responses of patients with breast cancer to chemotherapy.
Diffusion-weighted magnetic resonance (MR) imaging detects Brownian motion of water protons, thus reflecting the biologic character of tissue. The apparent diffusion coefficient (ADC) is used to quantify the Brownian motion. Diffusion-weighted MR imaging detects early changes in the morphology and physiology of tissues associated with changes in water content, such as changes in the permeability of cell membranes, cell swelling, and/or cell lysis. Areas of diseased tissue are highlighted with increased signal intensity on diffusion-weighted MR imaging. A decrease in the ADC is expected with increased intracellular tissue caused by either cell swelling or increased cellular density . Diffusion-weighted imaging has a potential role for the characterization of breast mass and treatment monitoring after chemotherapy .
The aim of this work is to review the techniques and clinical applications of diffusion-weighted MR imaging of the breast.
Techniques
Diffusion-Weighted Technique
No consensus exists among different research groups regarding the best diffusion-weighted technique for the breast. Most groups perform diffusion-weighted imaging on the basis of an echo-planar imaging (EPI) approach. Other groups apply turbo spin-echo techniques. Although EPI is fast and has a high signal-to-noise ratio, it is distorted by susceptibility and chemical shift artifacts as well as breathing and other motion artifacts. Geometric distortion arises from susceptibility differences between tissues or at air-tissue interfaces. Parallel imaging such as sensitivity encoding reduces the number of phase encoding steps and the time required to fill the k-space, which leads to decreased susceptibility and chemical shift artifacts . The typical parameters of single-shot EPI are as follows: repetition time, 700 ms; echo time, 75 ms; thickness gap, 5 mm; number of signals acquired, 6; slice number, 25; b value, 500, and 1000 mm 2 /s; scan time, 2 minutes; field of view, 320 mm; matrix size, 256 × 128; and voxel size, 1.25 × 1.25 × 5 mm. Turbo spin-echo techniques, such as the half-Fourier acquisition single-shot turbo spin-echo sequence, can also be used to acquire diffusion-weighted data, with the inherent advantage of using spin echoes rather than gradient echoes. There are no chemical shift or susceptibility artifacts, but the ability to detect tumors is limited .
Fat Suppression Technique
One limitation of diffusion-weighted imaging of the breast is the high content of fatty tissue in the breast, which makes a fat saturation technique essential to identifying breast mass on breast diffusion-weighted MR imaging. The two main fat suppression techniques used are the short time inversion recovery and chemical shift selective suppression (CHESS) methods. The short time inversion recovery method applies 180° prepulse and provides steady fat suppression, but its signal-to-noise ratio is lower than that of CHESS. The CHESS method does not always provide uniform fat suppression, but its signal-to-noise ratio is higher. In the CHESS method, shimming during imaging is applied to control the nonuniform fat suppression effect . Diffusion-weighted MR imaging of the breast with both sequences has a high potential to differentiate between benign and malignant breast lesions. Because of significant better lesion delineation, better selectivity, and shorter acquisition time the diffusion-weighted EPI CHESS sequence is superior .
b Value
The optimum b value applied should sufficiently suppress the background signal of the mammary gland and provide adequate cancer signal without perfusion effect. ADCs with b values < 400 mm 2 /s are affected not only by the molecular diffusion of water but also by the microcirculation of blood in the capillary network. Because invasive ductal cancer has an increased number and size of capillaries, the ADC of invasive ductal cancer can be strongly affected by perfusion when the b value is small. The background and cancer signals are separated considerably when the b value is 750 mm 2 /s. As the b value increases up to 1000 mm 2 /s, the background signal decreases to near noise levels, whereas the cancer signal remains at a significant level. In severe mastopathy, the background mammary gland signal may not be suppressed sufficiently. The spread of cancers that develop mainly through breast ducts is not as restricted as that of invasive cancers. Therefore, if the b value is set too high, cancers may be detected in a reduced form .
MR Imaging at 3 T
Get Radiology Tree app to read full this article<
Effect of Contrast Medium
Get Radiology Tree app to read full this article<
Normal appearance
Get Radiology Tree app to read full this article<
Clinical applications
Differentiation of Breast Cancer From Benign Lesions
Get Radiology Tree app to read full this article<
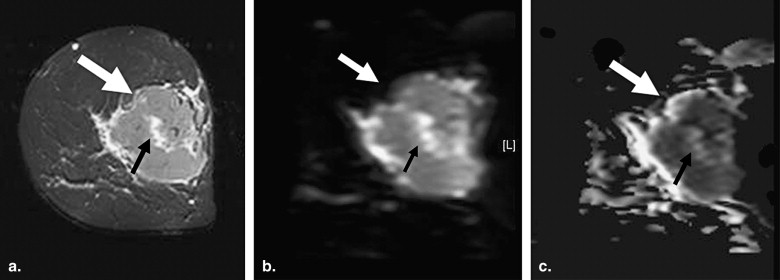
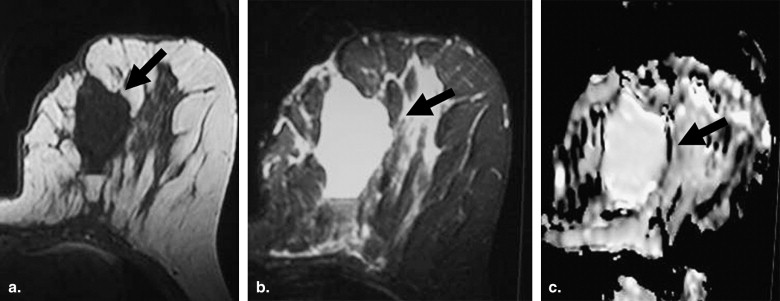
Characterization of Malignancy
Get Radiology Tree app to read full this article<
Ductal Carcinoma
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Mucinous Carcinoma
Get Radiology Tree app to read full this article<
Other Pathologic Types
Get Radiology Tree app to read full this article<
Peritumoral Spread
Get Radiology Tree app to read full this article<
Correlation of the ADC with Tumor Cellularity
Get Radiology Tree app to read full this article<
Correlation of the ADC with Prognostic Parameters
Get Radiology Tree app to read full this article<
Benign Breast Lesions
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Monitoring of Treatment Response
Get Radiology Tree app to read full this article<
Screening
Get Radiology Tree app to read full this article<
Advantages
Get Radiology Tree app to read full this article<
Disadvantages
Get Radiology Tree app to read full this article<
Conclusion
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
References
1. Neil J.: Diffusion imaging concepts for clinicians. J Magn Reson Imaging 2008; 27: pp. 1-8.
2. Koh D., Collins D.: Diffusion-weighted MRI in the body: applications and challenges in oncology. AJR Am J Roentgenol 2007; 88: pp. 1622-1635.
3. Tsushima Y., Takahashi-Taketomi A., Endo K.: Magnetic resonance (MR) differential diagnosis of breast tumors using apparent diffusion coefficient (ADC) on 1.5-T. J Magn Reson Imaging 2009; 30: pp. 249-255.
4. Sinha S., Sinha U.: Recent advances in breast MRI and MRS. NMR Biomed 2009; 22: pp. 3-16.
5. Kuroki Y., Nasu K.: Advances in breast MRI: diffusion-weighted imaging of the breast. Breast Cancer 2008; 15: pp. 212-217.
6. Partridge S.: Future applications and innovations of clinical breast magnetic resonance imaging. Top Magn Reson Imaging 2008; 19: pp. 171-176.
7. Kuroki Y., Nasu K., Kuroki S., et. al.: Diffusion-weighted imaging of breast cancer with the sensitivity encoding technique: analysis of the apparent diffusion coefficient value. Magn Reson Med Sci 2004; 3: pp. 79-85.
8. Wenkel E., Geppert C., Schulz-Wendtland R., et. al.: Diffusion weighted imaging in breast MRI: comparison of two different pulse sequences. Acad Radiol 2007; 14: pp. 1077-1083.
9. Baltzer P., Renz D., Herrmann K., et. al.: Diffusion-weighted imaging (DWI) in MR mammography (MRM): clinical comparison of echo planar imaging (EPI) and half-Fourier single-shot turbo spin echo (HASTE) diffusion techniques. Eur Radiol 2009; 19: pp. 1612-1620.
10. Kuroki-Suzuki S., Kuroki Y., Nasu K., et. al.: Detecting breast cancer with non-contrast MR: combining diffusion-weighted and STIR imaging. Magn Reson Med Sci 2007; 6: pp. 21-27.
11. Lo G.G., Ai V., Chan J.K., et. al.: Diffusion-weighted magnetic resonance imaging of breast lesions: first experiences at 3 T. J Comput Assist Tomogr 2009; 33: pp. 63-69.
12. Matsuoka A., Minato M., Harada M., et. al.: Comparison of 3.0- and 1.5-tesla diffusion-weighted imaging in the visibility of breast cancer. Radiat Med 2008; 26: pp. 15-20.
13. Yuen S., Yamada K., Goto M., et. al.: Microperfusion-induced elevation of ADC is suppressed after contrast in breast carcinoma. J Magn Reson Imaging 2009; 29: pp. 1080-1084.
14. Partridge S.C., McKinnon G.C., Henry R.G., et. al.: Menstrual cycle variation of apparent diffusion coefficients measured in the normal breast using MRI. J Magn Reson Imaging 2001; 14: pp. 433-438.
14. Marini C., Iacconi C., Giannelli M., et. al.: Qualitative diffusion-weighted MR imaging in the differential diagnosis of breast lesions. Eur Radiol 2007; 17: pp. 2646-2655.
15. Wiratkapun C., Duke D., Nordmann A.S., et. al.: Indeterminate or suspicious breast lesions detected initially with MR imaging: value of MRI-directed breast ultrasound. Acad Radiol 2008; 15: pp. 618-625.
16. Renz D.M., Baltzer P.A.T., Bottcher J., et. al.: Inflammatory breast carcinoma in magnetic resonance imaging: a comparison with locally advanced breast cancer. Acad Radiol 2008; 15: pp. 209-221.
17. Rajagopal V., Lee A., Chung J.H., et. al.: Creating individual-specific biomechanical models of the breast for medical image analysis. Acad Radiol 2008; 15: pp. 1425-1436.
18. Woodhams R., Matsunaga K., Kan S., et. al.: ADC mapping of benign and malignant breast tumors. Magn Reson Med Sci 2005; 4: pp. 35-42.
19. Guo Y., Cai Y.Q., Cai Z.L., et. al.: Differentiation of clinically benign and malignant breast lesions using diffusion weighted imaging. J Mag Reson Imaging 2002; 16: pp. 172-178.
20. Sinha S., Lucas-Quesada F.A., Sinha U., et. al.: In vivo diffusion-weighted MRI of the breast: potential for lesion characterization. J Mag Reson Imaging 2002; 15: pp. 693-704.
21. Kinoshita T., Yashiro N., Ihara N., et. al.: Diffusion-weighted half-Fourier single-shot turbo spin echo imaging in breast tumors: differentiation of invasive ductal carcinoma from fibroadenoma. J Comput Assist Tomogr 2002; 26: pp. 1042-1046.
22. Rubesova E., Grell A.S., De Maertelaer V., et. al.: Quantitative diffusion imaging in breast cancer: a clinical prospective study. J Magn Reson Imaging 2006; 24: pp. 319-324.
23. Park M.J., Cha E.S., Kang B.J., et. al.: The role of diffusion-weighted imaging and the apparent diffusion coefficient (ADC) values for breast tumors. Korean J Radiol 2007; 8: pp. 390-396.
24. Woodhams R., Kakita S., Hata H., et. al.: Diffusion-weighted imaging of mucinous carcinoma of the breast: evaluation of apparent diffusion coefficient and signal intensity in correlation with histologic findings. AJR Am J Roentgenol 2009; 193: pp. 260-266.
25. Woodhams R., Matsunaga K., Iwabuchi K., et. al.: Diffusion-weighted imaging of malignant breast tumors: the usefulness of apparent diffusion coefficient (ADC) value and ADC map for the detection of malignant breast tumors and evaluation of cancer extension. J Comput Assist Tomogr 2005; 29: pp. 644-649.
26. Yili Z., Xiaoyan H., Hongwen D., et. al.: The value of diffusion-weighted imaging in assessing the ADC changes of tissues adjacent to breast carcinoma. BMC Cancer 2009; 9: pp. 18.
27. Hatakenaka M., Soeda H., Yabuuchi H., et. al.: Apparent diffusion coefficients of breast tumors: clinical application. Magn Reson Med Sci 2008; 7: pp. 23-29.
28. Yoshikawa M., Ohsumi S., Sugata S., et. al.: Relation between cancer cellularity and apparent diffusion coefficient values using diffusion-weighted magnetic resonance imaging in breast cancer. Radiat Med 2008; 26: pp. 222-226.
29. Kim S., Cha E., Kim H., et. al.: Diffusion-weighted imaging of breast cancer: correlation of the apparent diffusion coefficient value with prognostic factors. J Magn Reson Imaging 2009; 30: pp. 615-620.
30. Pickles M.D., Gibbs P., Lowry M., et. al.: Diffusion changes precede size reduction in neoadjuvant treatment of breast cancer. Mag Reson Imag 2006; 24: pp. 843-847.
31. Yankeelov T., Lepage M., Chakravarthy A., et. al.: Integration of quantitative DCE-MRI and ADC mapping to monitor treatment response in human breast cancer: initial results. Mag Reson Imag 2007; 25: pp. 1-13.
32. Sharma U., Danishad K.K., Seenu V., et. al.: Longitudinal study of the assessment by MRI and diffusion-weighted imaging of tumor response in patients with locally advanced breast cancer undergoing neoadjuvant chemotherapy. NMR Biomed 2009; 22: pp. 104-113.