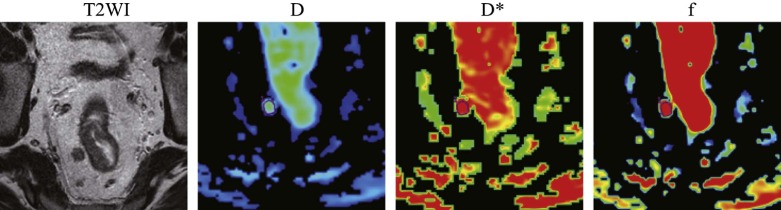
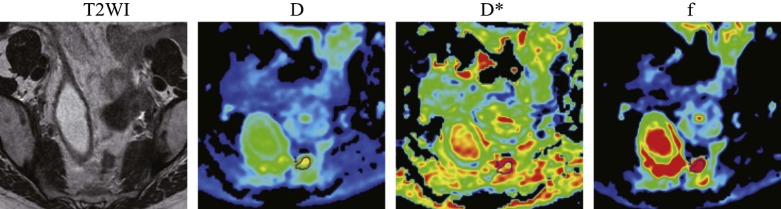
Rationale and Objectives
The aim of the study was to investigate the diagnostic value of intravoxel incoherent motion diffusion-weighted magnetic resonance imaging (IVIM DWI) for discriminating nonmetastatic from metastatic mesorectal lymph nodes in rectal cancer.
Materials and Methods
IVIM DWI was performed preoperatively on 50 patients with rectal carcinoma. The short-axis diameter, short- to long-axis diameter ratio, and IVIM-based parameter (pure diffusion coefficient [D], pseudo-diffusion coefficient [D*] and perfusion fraction [ f ]) values were compared between the metastatic and nonmetastatic lymph node groups.
Results
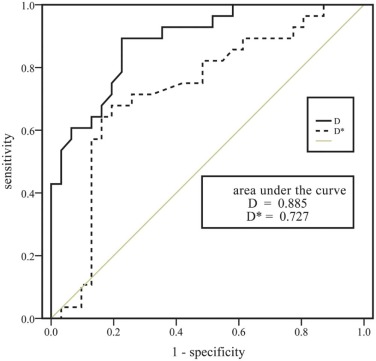
The short-axis diameter; short- to long-axis diameter ratio; and D, D*, and f values for the nonmetastatic lymph node group ( n = 28) were 6.446 ± 1.201 mm, 0.815 ± 0.099, 1.071 ± 0.234 × 10 −3 mm 2 /s, 15.443 ± 5.946 mm 2 /s and 0.261 ± 0.128, respectively, and were 9.045 ± 3.185 mm, 0.809 ± 0.099, 0.816 ± 0.121 × 10 −3 mm 2 /s, 11.679 ± 7.521 × 10 −3 mm 2 /s, and 0.190 ± 0.064, respectively, for the metastatic lymph node group ( n = 31). The short-axis diameter for the metastatic group was significantly higher than for the nonmetastatic group ( P < 0.001). The metastatic group exhibited significantly lower D and D* values than the nonmetastatic group ( P < 0.01). The short- to long-axis diameter ratio and f values did not differ significantly between the two groups. Optimal cutoff values (area under the curve, sensitivity, and specificity) for distinguishing metastatic from nonmetastatic lymph nodes were as follows: short-axis diameter = 5.563 mm (0.783, 74.2%, 82.1%); D = 0.667 × 10 −3 mm 2 /s (0.885, 77.4%, 89.3%); and D* = 0.485 × 10 −3 mm 2 /s (0.727, 80.6%, 67.9%).
Conclusion
IVIM DWI is useful to differentiate between metastatic and nonmetastatic mesorectal lymph nodes in rectal cancer.
As one of the most common malignant tumors in western countries, colorectal carcinoma is associated with high rates of morbidity and mortality . Lymph node staging in patients with rectal cancer is important for determining the method of surgical excision and whether the patient requires neoadjuvant chemoradiotherapy . Therefore, accurate lymph node staging is essential for optimizing individual treatment regimens. However, preoperative detection of lymph node involvement is always highly challenging for radiologists. Currently, magnetic resonance imaging (MRI) is often used to distinguish metastatic from nonmetastatic lymph nodes in patients with rectal cancer. Differential diagnosis of nodal involvement on conventional MRI is based on the size and shape of the lymph nodes, the presence of capsular involvement and central necrosis, and inhomogeneous enhancement after intravenous administration of a contrast agent . However, the accuracy of discrimination based on morphology-based MRI could be improved. This might be, at least in part, attributed to the fact that morphology-based MRI provides limited useful functional information on tissues, such as perfusion and diffusion information.
Based on an evaluation of the diffusion mobility of water molecules, diffusion-weighted magnetic resonance imaging (DWI) is a well-established functional MRI technique that can reveal the diffusion characteristics of tissues. Several recent studies have reported inconsistent findings on the ability of DWI to determine lymph node status in rectal cancer . For example, Cho et al. found that the apparent diffusion coefficient (ADC) value for metastatic lymph nodes was significantly lower than for nonmetastatic lymph nodes in rectal cancer, and the ADC value could be used to discriminate metastatic and nonmetastatic lymph nodes with a diagnostic accuracy of approximate 70%. By contrast, Heijnen et al. reported that benign and malignant nodes exhibited similar ADC values. These conflicting results might be partly due to the fact that the conventional DWI used in the previously mentioned studies is based on a mono-exponential model using data at b = 0 s/mm 2 and another b value other than zero and ignores the influence of microcirculation perfusion on the signal intensity of diffusion on DWI. Therefore, the conventional DWI parameter (ADC) cannot accurately reflect the diffusion characteristics of tissues. Based on the biexponential model developed by Le Bihan et al. , intravoxel incoherent motion (IVIM) DWI can simultaneously obtain diffusion and perfusion information on tissues. IVIM DWI has been widely used to determine the microenvironmental features of primary tumors and metastatic lymph nodes , discern the primary malignancies from postchemoradiation fibrosis , and differentiate benign from malignant tumors . Nevertheless, the usefulness of IVIM DWI in identifying nodal involvement in patients with rectal cancer is not clear. Therefore, we hypothesized that IVIM-based parameter values would differ between the metastatic and nonmetastatic mesorectal lymph nodes in patients with rectal cancer. In the present study, we aimed to explore the diagnostic efficacy of IVIM DWI for the differentiation between the two types of lymph nodes.
Materials and Methods
Patient Selection
Get Radiology Tree app to read full this article<
Conventional MRI Protocol
Get Radiology Tree app to read full this article<
IVIM DWI Protocol
Get Radiology Tree app to read full this article<
IVIM Analysis
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Sb/S0=(1−f)exp(−bD)+fexp(−b[D*+D]) S
b
/
S
0
=
(
1
−
f
)
exp
(
−
bD
)
+
f
exp
(
−
b
[
D
*
+
D
]
)
where S b is the signal intensity with diffusion gradient b, S 0 is the signal intensity for a b value of 0 s/mm 2 , D is the true diffusion coefficient (in mm 2 /s) indicating the pure diffusion of the water molecules, f is the microvascular volume fraction representing the fraction of diffusion related to microcirculation perfusion, and D* is the pseudo-diffusion coefficient (in mm 2 /s) demonstrating microcirculation perfusion. Because D* is roughly one order of magnitude greater than D , −bD* is less than −3 at a high b value (>200 s/mm 2 ) and f exp(−bD*) is less than 0.05 f . In this case, the contribution of D* to the S b /S 0 signal ratio can be ignored, and Equation (1) can be simplified to Equation (2) to estimate D:
Sb/S0=exp(−bD) S
b
/
S
0
=
exp
(
−
bD
)
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Histopathology
Get Radiology Tree app to read full this article<
Statistical Analysis
Get Radiology Tree app to read full this article<
Results
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Table 1
Comparison of the Size, Shape, and IVIM-based Parameter Values (Mean ± Standard Deviation) between the Metastatic and Nonmetastatic Groups
Parameters Nonmetastatic group ( n = 28) Metastatic group ( n = 31)P Value Short-axis diameter (mm) 6.446 ± 1.201 9.045 ± 3.185 # 0.000 Short- to long-axis diameter ratio 0.815 ± 0.099 0.809 ± 0.099 0.838 D (×10 −3 mm 2 /s) 1.071 ± 0.234 0.816 ± 0.121 # 0.000 D* (×10 −3 mm 2 /s) 15.443 ± 5.946 11.679 ± 7.521 # 0.003f 0.261 ± 0.128 0.190 ± 0.064 0.051
IVIM, intravoxel incoherent motion; D, pure diffusion coefficient; D*, pseudo-diffusion coefficient; f , perfusion fraction.
Get Radiology Tree app to read full this article<
Table 2
Optimal Cutoff Values for Differentiation between the Metastatic and Nonmetastatic Groups Based on Receiver-operating Characteristic Curve Analysis
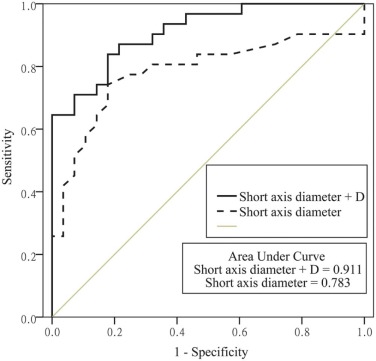
Parameters Cutoff Value AUC (95% CI) Sensitivity Specificity_P_ Value Short-axis diameter 5.563 mm 0.783 (0.658–0.908) 74.19% 82.14% 0.148 a , 0.474 b D 0.667 × 10 −3 mm 2 /s 0.885 (0.804–0.967) 77.42% 89.29% 0.026 c D* 0.485 × 10 −3 mm 2 /s 0.727 (0.592–0.862) 80.65% 67.86% # 0.007 d Short-axis diameter + D 0.911 (0.842–0.981) 83.87% 82.14% 0.261 e , 0.027 f
AUC, area under the curve; CI, confidence interval; D, pure diffusion coefficient; D*, pseudo-diffusion coefficient. a, short-axis diameter vs D; b, short-axis diameter vs D*; c, D vs D*; d, D* vs short-axis diameter + D; e, short-axis diameter + D vs D; f, short-axis diameter + D vs short-axis diameter.
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Discussion
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Conclusions
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
References
1. American Cancer Society : Cancer facts and figs.2012.American Cancer SocietyAtlanta, GA www.cancer.gov
2. Suzuki K., Muto T., Sawada T.: Prevention of local recurrence by extended lymphadenectomy for rectal cancer. Surg Today 1995; 25: pp. 795-801.
3. King A.D., Tse G.M., Ahuja A.T., et. al.: Necrosis in metastatic neck nodes: diagnostic accuracy of CT, MR imaging, and US. Radiology 2004; 230: pp. 720-726.
4. Cho E.Y., Kim S.H., Yoon J.H., et. al.: Apparent diffusion coefficient for discriminating metastatic from non-metastatic lymph nodes in primary rectal cancer. Eur J Radiol 2013; 82: pp. e662-e668.
5. Heijnen L.A., Lambregts D.M., Mondal D., et. al.: Diffusion-weighted MR imaging in primary rectal cancer staging demonstrates but does not characterise lymph nodes. Eur Radiol 2013; 23: pp. 3354-3360.
6. Le Bihan D., Turner R.: The capillary network: a link between IVIM and classical perfusion. Magn Reson Med 1992; 27: pp. 171-178.
7. Lu Y., Jansen J.F., Stambuk H.E., et. al.: Comparing primary tumors and metastatic nodes in head and neck cancer using intravoxel incoherent motion imaging: a preliminary experience. J Comput Assist Tomogr 2013; 37: pp. 346-352.
8. Lai V., Li X., Lee V.H., et. al.: Intravoxel incoherent motion MR imaging: comparison of diffusion and perfusion characteristics between nasopharyngeal carcinoma and post-chemoradiation fibrosis. Eur Radiol 2013; 23: pp. 2793-2801.
9. Sigmund E.E., Cho G.Y., Kim S., et. al.: Intravoxel incoherent motion imaging of tumor microenvironment in locally advanced breast cancer. Magn Reson Med 2011; 65: pp. 1437-1447.
10. Beets-Tan R.G., Lambregts D.M., Maas M., et. al.: Magnetic resonance imaging for the clinical management of rectal cancer patients: recommendations from the 2012 European Society of Gastrointestinal and Abdominal Radiology (ESGAR) consensus meeting. Eur Radiol 2013; 23: pp. 2522-2531.
11. Nougaret S., Reinhold C., Mikhael H.W., et. al.: The use of MR imaging in treatment planning for patients with rectal carcinoma: have you checked the “DISTANCE”?. Radiology 2013; 268: pp. 330-344.
12. Marquardt D.: An algorithm for least-squares estimation of nonlinear parameters. J Soc Indust Appl Math 1963; 11: pp. 431-441.
13. Le Bihan D., Breton E., Lallemand D., et. al.: Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology 1988; 168: pp. 497-505.
14. Shim W.H., Kim H.S., Choi C.G., et. al.: Comparison of apparent diffusion coefficient and intravoxel incoherent motion for differentiating among glioblastoma, metastasis, and lymphoma focusing on diffusion-related parameter. PLoS ONE 2015; 10: pp. e0134761.
15. Gloria C., Li Q., Xu L., et. al.: Differentiation of diffusion coefficients to distinguish malignant and benign tumor. J Xray Sci Technol 2010; 18: pp. 235-249.
16. Fong D., Bhatia K.S., Yeung D., et. al.: Diagnostic accuracy of diffusion-weighted MR imaging for nasopharyngeal carcinoma, head and neck lymphoma and squamous cell carcinoma at the primary site. Oral Oncol 2010; 46: pp. 603-606.
17. Zhang S.X., Jia Q.J., Zhang Z.P., et. al.: Intravoxel incoherent motion MRI: emerging applications for nasopharyngeal carcinoma at the primary site. Eur Radiol 2014; 24: pp. 1998-2004.
18. Jenkinson M.D., du Plessis D.G., Smith T.S., et. al.: Cellularity and apparent diffusion coefficient in oligodendroglial tumours characterized by genotype. J Neurooncol 2010; 96: pp. 385-392.
19. White M.L., Zhang Y., Robinson R.A.: Evaluating tumors and tumorlike lesions of the nasal cavity, the paranasal sinuses, and the adjacent skull base with diffusion-weighted MRI. J Comput Assist Tomogr 2006; 30: pp. 490-495.
20. Sumi M., Van Cauteren M., Sumi T., et. al.: Salivary gland tumors: use of intravoxel incoherent motion MR imaging for assessment of diffusion and perfusion for the differentiation of benign from malignant tumors. Radiology 2012; 263: pp. 770-777.
21. Pang Y., Turkbey B., Bernardo M., et. al.: Intravoxel incoherent motion MR imaging for prostate cancer: an evaluation of perfusion fraction and diffusion coefficient derived from different b-value combinations. Magn Reson Med 2013; 69: pp. 553-562.
22. Chen Y.B., Liao J., Xie R., et. al.: Discrimination of metastatic from hyperplastic pelvic lymph nodes in patients with cervical cancer by diffusion-weighted magnetic resonance imaging. Abdom Imaging 2011; 36: pp. 102-109.
23. Fischbein N.J., Noworolski S.M., Henry R.G., et. al.: Assessment of metastatic cervical adenopathy using dynamic contrast-enhanced MR imaging. AJNR Am J Neuroradiol 2003; 24: pp. 301-311.
24. Kvistad K.A., Rydland J., Smethurst H.B., et. al.: Axillary lymph node metastases in breast cancer: preoperative detection with dynamic contrast-enhanced MRI. Eur Radiol 2000; 10: pp. 1464-1471.
25. Murray A.D., Staff R.T., Redpath T.W., et. al.: Dynamic contrast enhanced MRI of the axilla in women with breast cancer: comparison with pathology of excised nodes. Br J Radiol 2002; 75: pp. 220-228.
26. Lewin M., Fartoux L., Vignaud A., et. al.: The diffusion-weighted imaging perfusion fraction f is a potential marker of sorafenib treatment in advanced hepatocellular carcinoma: a pilot study. Eur Radiol 2011; 21: pp. 281-290.
27. Wang L.L., Lin J., Liu K., et. al.: Intravoxel incoherent motion diffusion-weighted MR imaging in differentiation of lung cancer from obstructive lung consolidation: comparison and correlation with pharmacokinetic analysis from dynamic contrast-enhanced MR imaging. Eur Radiol 2014; 24: pp. 1914-1922.
28. Liu X., Peng W., Zhou L., et. al.: Biexponential apparent diffusion coefficients values in the prostate: comparison among normal tissue, prostate cancer, benign prostatic hyperplasia and prostatitis. Korean J Radiol 2013; 14: pp. 222-232.
29. Lemke A., Laun F.B., Simon D., et. al.: An in vivo verification of the intravoxel incoherent motion effect in diffusion-weighted imaging of the abdomen. Magn Reson Med 2010; 64: pp. 1580-1585.
30. Brown G., Richards C.J., Bourne M.W., et. al.: Morphologic predictors of lymph node status in rectal cancer with use of high-spatial-resolution MR imaging with histopathologic comparison. Radiology 2003; 227: pp. 371-377.
31. Arii K., Takifuji K., Yokoyama S., et. al.: Preoperative evaluation of pelvic lateral lymph node of patients with lower rectal cancer: comparison study of MR imaging and CT in 53 patients. Langenbecks Arch Surg 2006; 391: pp. 449-454.
32. Zerhouni E.A., Rutter C., Hamilton S.R., et. al.: CT and MR imaging in the staging of colorectal carcinoma: report of the Radiology Diagnostic Oncology Group II. Radiology 1996; 200: pp. 443-451.
33. Bipat S., Glas A.S., Slors F.J., et. al.: Rectal cancer: local staging and assessment of lymph node involvement with endoluminal US, CT, and MR imaging—a meta-analysis. Radiology 2004; 232: pp. 773-783.
34. Moffat B.A., Chenevert T.L., Meyer C.R., et. al.: The functional diffusion map: an imaging biomarker for the early prediction of cancer treatment outcome. Neoplasia 2006; 8: pp. 259-267.
35. Ungersma S.E., Pacheco G., Ho C., et. al.: Vessel imaging with viable tumor analysis for quantification of tumor angiogenesis. Magn Reson Med 2010; 63: pp. 1637-1647.
36. Kim J.H., Im G.H., Yang J., et. al.: Quantitative dynamic contrast-enhanced MRI for mouse models using automatic detection of the arterial input function. NMR Biomed 2012; 25: pp. 674-684.
37. Günther K., Dworak O., Remke S., et. al.: Prediction of distant metastases after curative surgery for rectal cancer. J Surg Res 2002; 103: pp. 68-78.
38. Hildebrandt U., Klein T., Feifel G., et. al.: Endosonography of pararectal lymph nodes. In vitro and in vivo evaluation. Dis Colon Rectum 1990; 33: pp. 863-868.