Rationale and Objectives
To determine the impact of second-opinion assessment on cancer staging and patient management in patients with pancreatic ductal adenocarcinoma.
Methods and Materials
This retrospective study was approved by our institutional review board with a waiver of informed consent. Second-opinion reports between January 1, 2009 and December 31, 2013, alongside outside reports for 65 consecutive cases of biopsy-proven pancreatic adenocarcinomas, were presented in random order to two experienced abdominal surgeons who independently reviewed them blinded to the origin of the report, images of the examinations, and patient identifier. Each surgeon filled in a questionnaire for each report recommending cancer staging and patient management. Recommended patient management and staging were evaluated against reference standards (actual patient management at 6 months following second-opinion assessment, and pathology or other clinical and imaging reference standards at 6 months or longer, respectively) using Cohen kappa.
Results
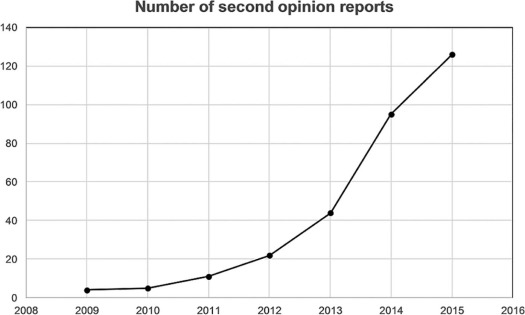
Cancer staging differed in 13% (9 of 65) of cases for surgeon 1 and in 18.4% (12 of 65) for surgeon 2. Patient management changed in 38.4% (25 of 65) of cases for surgeon 1 and in 20% (13 of 65) for surgeon 2. When compared to the pathologic staging gold standard, second opinion was correct in 85.7% (six of seven) of the time for both surgeons. Recommended patient management from second-opinion reports showed good agreement with the reference standard (weighted k = 0.6467 [0.4014–0.892] and weighted k = 0.6262 [0.3954–0.857] for surgeon 2).
Conclusion
Second-opinion review by subspecialized oncologic radiologists can impact patient care, specifically in terms of management decision.
Introduction
The increasing complexity of medical imaging has led to organ or disease subspecialization within the subfields of radiology. One of the latest subspecialties to emerge is oncologic imaging . The discrepancy between two radiologists reporting on the same images is a phenomenon that has been known since 1949 . Different studies have reported clear clinical benefits in introducing second-opinion imaging review in abdominal radiology, gynecolocical imaging and neuroradiology . In most clinical practices, it is not possible for every imaging study to be interpreted by a radiologist with subspecialty training. In this setting, general radiologists often fill the gap of subspecialty radiologists. However, it is common practice at many tertiary referral institutions for clinicians and surgeons to request second-opinion review of images by subspecialty radiologists, most of the time without writing a new formal report. This is possible because no new imaging is needed, but only new image interpretation . This informal radiologic opinion is often reported in the patients’ chart by the clinicians and surgeons, but without the chance for the second-opinion interpreting radiologist to review it. Furthermore, there is no reimbursement for this type of subspecialty second opinion to the interpreting radiologist. Another type of second opinion is asked for during or before multidisciplinary disease management team (DMT) meetings. Nowadays, many tertiary referral centers rely on DMTs for discussion of management of different types of cancers. During these meetings, outside radiologic examinations are reviewed by a subspecialty-trained radiologist without the issue of a formal report, often changing the management of the disease. In case of other specialties such as surgery or clinical specialties, a second-opinion practice is commonly accepted and reimbursed, whereas the rationale for reimbursing radiologic reassessments and for new reports issuing is still under debate . Multiple studies have reported a 1%–20% discrepancy in image interpretation . A disease-based formal study is needed to assess the benefits of such second-opinion reads in terms of changes in patient management.
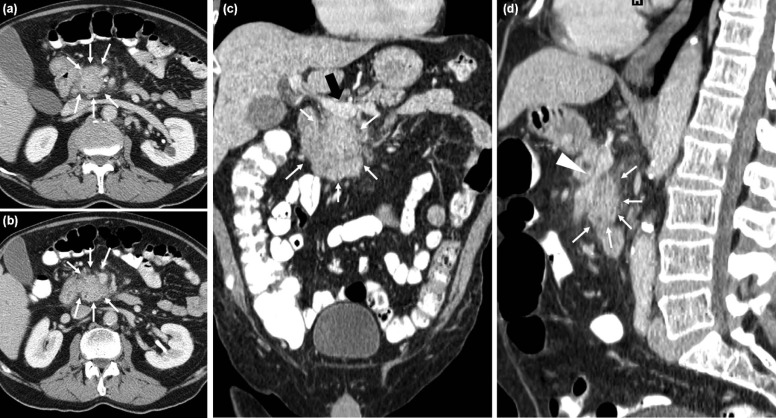
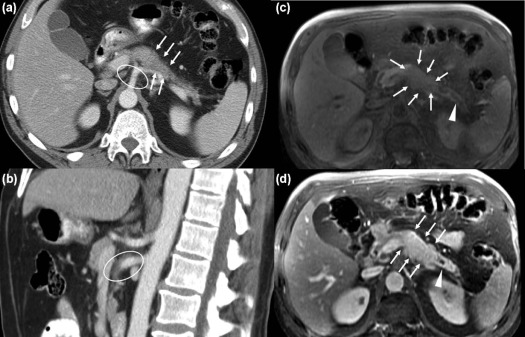
Our study focused on biologically proven pancreatic ductal adenocarcinoma (PDAC), one of the most lethal cancers with an overall survival at 5 years as low as 5% in the United States . We focused on a single type of cancer because of the lack of literature regarding second-opinion imaging review of this cancer and because pancreatic cancer treatment is heavily dependent on staging, which has a pivotal role in deciding the management of patients with PDAC. Additionally, a significant difference in short- and long-term outcomes has been noted when a pancreatectomy is performed at high-volume PDAC treatment centers . Many reasons can account for this better long-term outcome, it might be related to better surgeon skills as well as to the fact that subspecialized radiologists might provide better assessment of tumors in high-volume cancer centers. Lastly, general radiologists who often stage this type of cancer may not have the adequate expertise for interpreting all the different imaging patterns that pancreatic cancer can present on cross-sectional imaging, particularly when related with vascular involvement by the tumor (abutment, encasement, vessel deformity, and venous teardrop deformity) . Pancreatic cancers are approached by surgeons with a three-way decision model: resectable, borderline resectable, unresectable . According to TNM stage, stages I and II are resectable by definition because the tumor is confined within the pancreas. Stage IV is unresectable because it is characterized by distal metastases. Stage III is the most susceptible to radiologic interpretation mistakes, and it is defined as localized tumor with involvement of a major artery: celiac trunk, common hepatic artery, and superior mesenteric artery. It is further divided into two categories—locally advanced unresectable and borderline resectable. A borderline resectable tumor is defined by a contact between the tumor and the arterial circumference of no more than 180°. However, this definition of borderline resectable is not universally accepted and it might change among institutions, depending on the vessel involved, surgical skills and experience, and on patient status . When the tumor surrounds the arterial circumference for more than 180°, the vessel is considered encased and the tumor unresectable .
Get Radiology Tree app to read full this article<
Materials and Methods
Get Radiology Tree app to read full this article<
Inclusion Criteria
Get Radiology Tree app to read full this article<
TABLE 1
Population Characteristics and Inclusion Criteria
Population Characteristics and Patient Selection_n_ Total number of patients after database search 307 Number of patients in the time frame considered (01/2009–12/2013) 86 Patients excluded because of extra clinical information available (eg, surgery performed before the study started) 15 Patients excluded because of lack of follow-up 6 Final number of patient considered (M:F) 65 (39:26) Total number of CT examinations 51 Total number of MRI examinations 14
CT, computed tomography; MRI, magnetic resonance imaging; n , number.
Get Radiology Tree app to read full this article<
Second-opinion Assessment
Get Radiology Tree app to read full this article<
Retrospective Data Interpretation
Get Radiology Tree app to read full this article<
TABLE 2
Questionnaire to Fill in Provided by the Two Junior Radiologists to Two Oncologic Surgeons
Item Response1. Further imaging recommended to better evaluate an area or finding? (Circle one) Y N2. Resectability status (Circle one)
3. Stage (Circle one) I IIa IIb III IV4. Management (Circle one)
5. Grade of report (Circle one) Poor Excellent
1 2 3 4 5
The two surgeons were asked whether they needed further imaging studies to propose a certain management on the sole basis of the reports (if yes, they were asked about resectability status, staging according to the TNM staging system, and recommended management), and whether they were satisfied with the report on a scale from one to five.
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Statistical Analysis
Get Radiology Tree app to read full this article<
Results
Get Radiology Tree app to read full this article<
Review by Surgeons—Combined Results
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Review by Surgeons—Individual Results
Get Radiology Tree app to read full this article<
TABLE 3
Results of Questionnaire Filled in by Surgeons, Question 1: Number of Further Imaging Scan Examinations Asked by Surgeons Based on the Reports (Percentage and 95% Confidence Interval)
Surgeon 1 Surgeon 2 No further imaging scans asked based on the reports 27/65 (41.5% CI: 29.6–54.4) 27/65 (41.5% CI: 29.6–54.4) More imaging asked based on outside reports 21/65 (32.3% CI: 22.2–44.3) 9/65 (13.8% CI: 7.4–24.2) More imaging asked based on second reader reports 3/65 (4.6% CI: 1.5–12.7) 2/65 (3% CI: 0.8–10.5) More imaging asked for both reports 14/65 (21.5% CI: 13.2–32.9) 3/65 (4.6% CI: 1.5–12.7)
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
TABLE 4
This Table Shows How Surgeons Changed the Management of the Patients After Reading Second-opinion Reports, Compared to What Was Proposed After Reading Outside Reports: Number of Cases (Percentage and 95% Confidence Interval)
Surgeon 1 Surgeon 2 Concordance between outside and second reader reports 44/65 (67.6% CI: 55.6–77.9) 52/65 (80% CI: 68.7–87.9) Change of therapy: from surgery to chemotherapy 15/65 (23.1% CI: 14.5–34.6) 6/65 (9.2% CI: 0.4–18.7) Change of therapy: from chemotherapy to surgery 0/65 (0% CI: 0–0.58) 4/65 (6.1% CI: 2.4–14.7) Change of therapy: from neoadjuvant chemotherapy to chemotherapy 5/65 (7% CI: 3.3–16.6) 1/65 (1.5% CI: 0.2–8.2) Change of therapy: from chemotherapy to neoadjuvant chemotherapy 1/65 (1.5% CI: 0.2–8.2) 2/65 (3% CI: 0.8–10.5)
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
TABLE 5
Agreement Between Three Categories of Patient Management Proposed by Surgeons Based on Outside and Second-opinion Reports, Compared to Actual Patient Management as Found on the Clinical Report System (Surgery Summaries, Pathology, Blood Tests, and Additional Imaging Examinations)
Patients’ Clinical Management After 6 Months From Radiologic Assessment Surgeon 1 Surgeon 2 Surgery Neoadjuvant Chemotherapy Chemotherapy Weighted Kappa (95% CI) Agreement Surgery Neoadjuvant t Chemotherapy Chemotherapy Weighted Kappa (95% CI) Agreement Management as proposed after reading outside reports Surgery 6 2 10 3 2 6 Neoadjuvant chemotherapy 1 0 8 0.426 (0.2489–0.6031) 3 0 7 0.4136 (0.2149–0.6129) Chemotherapy 0 0 38 1 0 43 Management as proposed after reading second-opinion reports Surgery 3 0 1 5 0 3 Neoadjuvant chemotherapy 3 2 7 0.6467 (0.4014–0.892) 2 2 9 0.6262 (0.3954–0.857) Chemotherapy 1 0 48 0 0 44
Weighted kappa with squared weights was used to assess agreement.
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Discussion
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
References
1. Hricak H., Yu K.K., Dudley A., et. al.: Subspecialty care in radiology: oncologic imaging. Acad Radiol 1998; 5: pp. S288-S290.
2. Garland L.H.: On the scientific evaluation of diagnostic procedures: presidential address thirty-fourth annual meeting of the radiological Society of North America. Radiology 1949; 52: pp. 309-328.
3. Bell M.E., Patel M.D.: The degree of abdominal imaging (AI) subspecialization of the reviewing radiologist significantly impacts the number of clinically relevant and incidental discrepancies identified during peer review of emergency after-hours body CT studies. Abdom Imaging 2014; 39: pp. 1114-1118.
4. Lindgren E.A., Patel M.D., Wu Q., et. al.: The clinical impact of subspecialized radiologist reinterpretation of abdominal imaging studies, with analysis of the types and relative frequency of interpretation discrepancies. Abdom Imaging 2014; 39: pp. 1119-1126.
5. Lakhman Y., D’Anastasi M., Micco M., et. al.: Second-opinion interpretations of gynecologic oncologic MRI examinations by sub-specialized radiologists influence patient care. Eur Radiol 2016; 26: pp. 2089-2098.
6. Zan E., Yousem D.M., Carone M., et. al.: Second-opinion consultations in neuroradiology. Radiology 2010; 255: pp. 135-141.
7. Kalbhen C., Yetter E., Olson M., et. al.: Assessing the resectability of pancreatic carcinoma: the value of reinterpreting abdominal CT performed at other institutions. AJR Am J Roentgenol 1998; 171: pp. 1571-1576.
8. Gillen S., Schuster T., Meyer Zum Buschenfelde C., et. al.: Preoperative/neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of response and resection percentages. PLoS Med 2010; 7: e1000267
9. Yoshioka R., Yasunaga H., Hasegawa K., et. al.: Impact of hospital volume on hospital mortality, length of stay and total costs after pancreaticoduodenectomy. Br J Surg 2014; 101: pp. 523-529.
10. Birkmeyer J.D., Stukel T.A., Siewers A.E., et. al.: Surgeon volume and operative mortality in the United States. N Engl J Med 2003; 349: pp. 2117-2127.
11. He X.Y., Yuan Y.Z.: Advances in pancreatic cancer research: moving towards early detection. World J Gastroenterol 2014; 20: pp. 11241-11248.
12. Belousova E., Karmazanovsky G., Kriger A., et. al.: Contrast-enhanced MDCT in patients with pancreatic neuroendocrine tumours: correlation with histological findings and diagnostic performance in differentiation between tumour grades. Clin Radiol 2017; 72: pp. 150-158.
13. Mannelli L., Nougaret S., Vargas H.A., et. al.: Advances in diffusion-weighted imaging. Radiol Clin North Am 2015; 53: pp. 569-581.
14. Vauthey J.N., Dixon E.: AHPBA/SSO/SSAT consensus conference on resectable and borderline resectable pancreatic cancer: rationale and overview of the conference. Ann Surg Oncol 2009; 16: pp. 1725-1726.
15. Al-Hawary M.M., Francis I.R., Chari S.T., et. al.: Pancreatic ductal adenocarcinoma radiology reporting template: consensus statement of the Society of Abdominal Radiology and the American Pancreatic Association. Radiology 2014; 270: pp. 248-260.
16. Zaky A.M., Wolfgang C.L., Weiss M.J., et. al.: Tumor-vessel relationships in pancreatic ductal adenocarcinoma at multidetector CT: different classification systems and their influence on treatment planning. Radiographics 2016; 37: pp. 93-112.
17. Tempero M.A., Malafa M.P., Al-Hawary M., et. al.: Pancreatic adenocarcinoma, version 2.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2017; 15: pp. 1028-1061.
18. Seufferlein T., Bachet J.B., Van Cutsem E., et. al.: Pancreatic adenocarcinoma: ESMO-ESDO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2012; 23: pp. vii33-vii40.
19. Newcombe R.G.: Two-sided confidence intervals for the single proportion: comparison of seven methods. Stat Med 1998; 17: pp. 857-872.
20. Siegel R., Naishadham D., Jemal A.: Cancer statistics, 2013. CA Cancer J Clin 2013; 63: pp. 11-30.
21. Jemal A., Siegel R., Ward E., et. al.: Cancer statistics, 2008. CA Cancer J Clin 2008; 58: pp. 71-96.
22. Güngör C., Hofmann B., Wolters-Eisfeld G., et. al.: Pancreatic cancer. Br J Pharmacol 2014; 171: pp. 849-858.
23. Tamm E.P., Balachandran A., Bhosale P.R., et. al.: Imaging of pancreatic adenocarcinoma: update on staging/resectability. Radiol Clin North Am 2012; 50: pp. 407-428.
24. Irie H., Honda H., Kaneko K., et. al.: Comparison of helical CT and MR imaging in detecting and staging small pancreatic adenocarcinoma. Abdom Imaging 1997; 22: pp. 429-433.
25. Tscholakoff D., Hricak H., Thoeni R., et. al.: MR imaging in the diagnosis of pancreatic disease. AJR Am J Roentgenol 1987; 148: pp. 703-709.
26. Raman S.P., Horton K.M., Fishman E.K.: Multimodality imaging of pancreatic cancer-computed tomography, magnetic resonance imaging, and positron emission tomography. Cancer J 2012; 18: pp. 511-522.
27. Liu Y.J., Smith-Chakmakova F., Rassaei N., et. al.: Frozen section interpretation of pancreatic margins: subspecialized gastrointestinal pathologists versus general pathologists. Int J Surg Pathol 2016; 24: pp. 108-115.
28. Walters D.M., LaPar D.J., de Lange E.E., et. al.: Pancreas-protocol imaging at a high-volume center leads to improved preoperative staging of pancreatic ductal adenocarcinoma. Ann Surg Oncol 2011; 18: pp. 2764-2771.
29. Megibow A.J., Zhou X.H., Rotterdam H., et. al.: Pancreatic adenocarcinoma: CT versus MR imaging in the evaluation of resectability—report of the Radiology Diagnostic Oncology Group. Radiology 1995; 195: pp. 327-332.
30. Powell D.K., Silberzweig J.E.: State of structured reporting in radiology, a survey. Acad Radiol 2015; 22: pp. 226-233.
31. Brook O.R., Brook A., Vollmer C.M., et. al.: Structured reporting of multiphasic CT for pancreatic cancer: potential effect on staging and surgical planning. Radiology 2015; 274: pp. 464-472.
32. Ulaner G.A., Mannelli L., Dunphy M.: Value of second-opinion review of outside institution PET-CT examinations. Nucl Med Commun 2017; 38: pp. 306-311.
33. Wibmer A., Vargas H.A., Donahue T.F., et. al.: Diagnosis of extracapsular extension of prostate cancer on prostate MRI: impact of second-opinion readings by subspecialized genitourinary oncologic radiologists. AJR Am J Roentgenol 2015; 205: pp. W73-W78.
34. Cao H.S.T., Balachandran A., Wang H., et. al.: Radiographic tumor–vein interface as a predictor of intraoperative, pathologic, and oncologic outcomes in resectable and borderline resectable pancreatic cancer. J Gastrointest Surg 2014; 18: pp. 269-278.