Rationale and Objectives
Computed tomography (CT) plays a crucial role in early assessment of patients with traumatic brain injury (TBI). Marshall and Rotterdam are the mostly used scoring systems, in which CT findings are grouped differently. We sought to determine the scoring system and initial CT findings predicting the death at hospital discharge (early death) in patients with TBI.
Materials and Methods
We included 245 consecutive adult patients with mild-to-severe TBI. Their initial CT and status at hospital discharge (dead or alive) were reviewed, and both CT scores were calculated. We examined whether each score was related to early death; compared the two scoring systems’ performance in predicting early death, and identified the CT findings that are independent predictors of early death.
Results
More deaths occurred among patients with higher Marshall and Rotterdam scores (both P < .05, Mann–Whitney U test). The areas under the receiver operating characteristic curve (AUCs) indicated that both scoring systems had similarly good discriminative power in predicting early death (Marshall, AUC = 0. 85 vs. Rotterdam, AUC = 0.85). Basal cistern absence (odds ratio [OR] = 771.5, P < .0001), positive midline shift (OR = 56.2, P = .0011), hemorrhagic mass volume ≥25 mL (OR = 12.9, P = .0065), and intraventricular or subarachnoid hemorrhage (OR = 3.8, P = .0395) were independent predictors of early death.
Conclusions
Both Marshall and Rotterdam scoring systems can be used to predict early death in patients with TBI. The performance of the Marshall score is at least equal to that of the Rotterdam score. Thus, although older, the Marshall score remains useful in predicting patients’ prognosis.
Because traumatic brain injury (TBI) is a leading cause of mortality and morbidity among young people worldwide, outcome prediction at admission is crucial for clinical decision making, resource allocation, and counseling of patients’ families.
Computed tomography (CT) currently plays an important role in the rapid assessment of patients with TBI in that it detects posttraumatic hemorrhagic lesions and allows selection of patients who require emergency neurosurgery. To predict the outcome of patients with TBI, two scoring systems that use initial CT findings but group them differently have been introduced: Marshall score in 1991 which was followed by Rotterdam score in 2005 in an attempt to improve the performance yield in predicting patients’ outcome. Both scoring systems are currently used widely in studies assessing patients with TBI either to show subject demographics or as independent predictor of patients’ outcome .
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Methods
Patients
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Table 1
General Characteristics of the Study Population ( n = 245)
Characteristic Patients n (%) GCS at ED ∗ 12 (3.9) Sex Male 165 (67.3) Female 80 (32.7) Age (years) ∗ 49.4 (22.6) Mechanism of injury Traffic accident 121 (49.4) Fall 106 (43.3) Other 18 (7.3) Discharge status Alive 220 (89.8) Dead 25 (10.2)
ED, emergency department; GCS, Glasgow coma scale.
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Patient Status at Hospital Discharge
Get Radiology Tree app to read full this article<
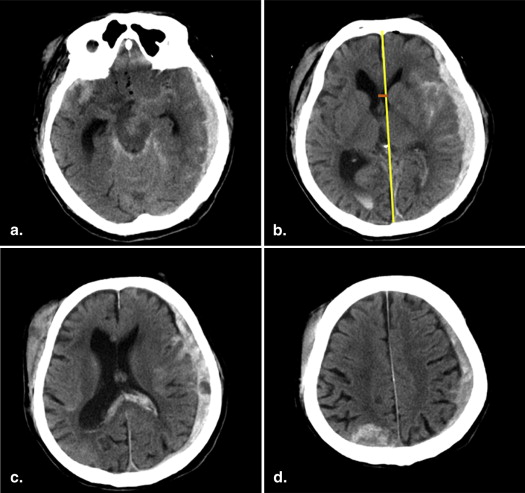
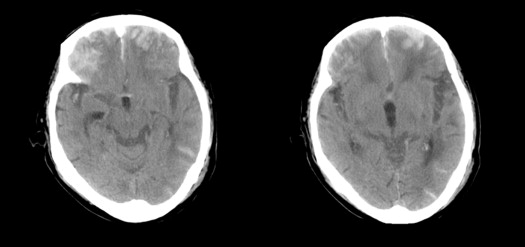
Evaluation of CT Findings
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Table 2
Five CT Items Included in the Marshall and/or Rotterdam Score
CT Finding Marshall Rotterdam Basal cistern status Included Included Midline shift Included Included EDH Not included Included SAH/IVH Not included Included Hemorrhagic mass volume Included Not included
CT, computed tomography; EDH, epidural hematoma; SAH/IVH, subarachnoid hemorrhage/intraventricular hemorrhage.
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Calculation of Marshall and Rotterdam scores
Get Radiology Tree app to read full this article<
Table 3
Marshall Scoring System
Adapted from Mass et al. .
Score Definition 1 No visible intracranial pathology on computed tomography 2 Cisterns are present with 0–5 mm midline shift and/or lesion densities present; no high- or mixed-density lesion >25 mL includes bone fragments or foreign bodies 3 Cisterns compressed or absent with 0–5 mm midline shift; no high- or mixed-density lesion >25 mL 4 Midline shift >5 mm; no high- or mixed-density lesion >25 mL 5 Any lesion surgically evacuated 6 High- or mixed-density lesion >25 mL; not surgically evacuated
Table 4
Rotterdam Scoring System
CT Finding Score Definition Basal cistern 0 Normal 1 Compressed 2 Absent Midline shift 0 ≤5 mm 1 >5 mm EDH 1 Absent 0 Present SAH/IVH 0 Absent 1 Present
EDH, epidural hematoma; SAH/IVH, subarachnoid hemorrhage/intraventricular hemorrhage.
The final score (0–6) is calculated by summing the item scores and adding 1.
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Statistical Analysis
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Results
Performance of Marshall and Rotterdam CT Scoring Systems in Predicting Early Death
Get Radiology Tree app to read full this article<
Table 5
Distribution of Patients ( n = 245) According to Marshall and Rotterdam Scores
Computed Tomography Scoring System Patients n (%) Marshall score 1 132 (53.9) 2 67 (27.4) 3 3 (1.2) 4 1 (0.4) 5 30 (12.2) 6 12 (4.9) Rotterdam score 1 2 (0.8) 2 149 (60.8) 3 62 (25.3) 4 11 (4.5) 5 14 (5.7) 6 7 (2.9)
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
CT Findings Independently Associated with Early Death
Get Radiology Tree app to read full this article<
Table 6
CT Findings Independently Predicting Early Death, Identified by Multiple Logistic Regressions
CT Finding Number of Patients (%) Nonadjusted OR_P_ Value Adjusted OR_P_ Value Basal cistern <.0001 ∗ <.0001 ∗ Normal 119 (85.7) 13.4 <.0001 ∗ 47.7 <.0001 ∗ Compressed 24 (9.8) 2.9 .1485 16.2 .0030 ∗ Absent 11 (4.5) 39.1 <.0001 ∗ 771.5 <.0001 ∗ Positive midline shift 31 (12.7) 7.9 <.0001 ∗ 56.2 .0011 ∗ Positive EDH 14 (5.7) 2.6 .2013 2.35 .3452 Positive SAH/IVH 89 (36.3) 8.8 <.0001 ∗ 3.8 .0395 ∗ Hemorrhagic mass <.0001 ∗ .0247 ∗ Absent 154 (62.8) 7.8 .0004 ∗ 2.6 .2123 <25 mL 58 (23.7) 2.4 .0842 4.9 .0564 ≥25 mL 33 (13.5) 18.7 <.0001 ∗ 12.9 .0065 ∗
CT, computed tomography; EDH, epidural hematoma; OR, odds ratio; SAH/IVH, subarachnoid hemorrhage/intraventricular hemorrhage.
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Discussion
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Conclusions
Get Radiology Tree app to read full this article<
References
1. Marshall L.F., Eisenberg H., Jane J.A., et. al.: A new classification of head injury based on computed tomography. J Neurosurg 1991; 75: pp. S14-S20.
2. Maas A.I., Hukkelhoven C.W., Marshall L.F., et. al.: Prediction of outcome in traumatic brain injury with computed tomographic characteristics: a comparison between the computed tomographic classification and combinations of computed tomographic predictors. Neurosurgery 2005; 57: pp. 1173-1182. discussion 1173–1182
3. Chun K.A., Manley G.T., Stiver S.I., et. al.: Interobserver variability in the assessment of CT imaging facture of traumatic brain injury. J Neurotrauma 2010; 27: pp. 325-330.
4. Hilarioa A., Ramos A., Millan J.M., et. al.: Severe traumatic head injury: prognostic value of brain stem injuries detected at MRI. AJNR Am J Neuroradiol 2012; 33: pp. 1925-1931.
5. Nelson D.W., Nyström H., MacCallum R.M., et. al.: Extended analysis of early computed tomography scans of traumatic brain injured patients and relations to outcome. J Neurotrauma 2010; 27: pp. 51-64.
6. Katsnelson M., Mackenzie L., Frangos S., et. al.: Are initial radiology and clinical scales associated with subsequent intracranial pressure and brain oxygen level after severe traumatic brain injury?. Neurosurgery 2012; 70: pp. 1095-1105.
7. Huang Y.H., Deng Y.H., Lee T.C., et. al.: Rotterdam computed tomography score as a prognosticator in head-injured patients undergoing decompressive craniectomy. Neurosurgery 2012; 71: pp. 80-85.
8. Haydel M.J., Preston C.A., Mills T.J., et. al.: Indications for computed tomography in patients with minor head injury. N Engl J Med 2000; 343: pp. 100-105.
9. Stiell I.G., Lesiuk H., Wells G.A., et. al.: Canadian CT head rule study for patients with minor head injury: methodology for phase II (validation and economic analysis). Ann Emerg Med 2001; 38: pp. 317-322.
10. Smits M., Dippel D.W., Nederkoorn P.J., et. al.: Minor head injury: CT-based strategies for management—a cost-effectiveness analysis. Radiology 2010; 254: pp. 532-540.
11. Stocchetti N., Croci M., Spagnoli D., et. al.: Mass volume measurement in severe head injury: accuracy and feasibility of two pragmatic methods. J Neurol Neurosurg Psychiatry 2000; 68: pp. 14-17.
12. Jacobs B., Beems T., Van der Vliet T.M., et. al.: Computed tomography and outcome in moderate and severe traumatic brain injury: hematoma volume and midline shift revisited. J Neurotrauma 2011; 28: pp. 203-215.
13. Maas A.I., Steyerberg E.W., Butcher I., et. al.: Prognostic value of computerized tomography scan characteristics in traumatic brain injury: results from the IMPACT study. J Neurotrauma 2007; 24: pp. 303-314.
14. Harrell FE. Regression modeling strategies: with applications to linear models, logistic regression, and survival analysis. Springer series in statistics, Springer-Verlag, New York, Inc, 2001.
15. Steyerberg E.W., Mushkudiani N., Perel P., et. al.: Predicting outcome after traumatic brain injury: development and international validation of prognostic scores based on admission characteristic. PLoS Med 2008; 5: pp. e165.
16. Lingsma H.F., Roozenbeek B., Steyerberg E.W., et. al.: Early prognosis in traumatic brain injury: from prophecies to predictions. Lancet Neurol 2010; 9: pp. 543-554.
17. Jacobs B., Beems T., Stulemeijer M., et. al.: Outcome prediction in mild traumatic brain injury: age and clinical variables are stronger predictors than CT abnormalities. J Neurotrauma 2010; 27: pp. 655-668.
18. Goldschlager T., Rosenfeld J.V., Winter C.D.: ‘Talk and die’ patients presenting to a major trauma centre over a 10 year period: a critical review. J Clin Neurosci 2007; 14: pp. 618-623.
19. National center for injury prevention and control: Report to congress on mild traumatic brain injury in the United States: steps to prevent serious public problem.2003.center for disease control and preventionAtlanta, GA
20. Lee H., Wintermark M., Gean A.D., et. al.: Focal lesions in acute mild traumatic brain injury and neurocognitive outcome: CT versus 3T MRI. J Neurotrauma 2008; 25: pp. 1049-1056.
![Figure 3, Showing the relationships between the Marshall (a) and Rotterdam (b) scores and early death, as determined by the Mann–Whitney U test. Marshall and Rotterdam scores were significantly higher among patients who were dead at the time of hospital discharge (median [lower, upper quartile]: Marshall, 5 [2, 6]; Rotterdam, 4 [3, 5]) than among those who were alive (Marshall, 1 [1, 2]; Rotterdam, 2 [2, 3]). Upper and lower bars indicate 75% and 25%, respectively.](https://storage.googleapis.com/dl.dentistrykey.com/clinical/EarlyCTFindingstoPredictEarlyDeathinPatientswithTraumaticBrainInjury/2_1s20S1076633214000464.jpg)