Rationale and Objectives
To determine the effect of radiofrequency (RF) ablation on normal lung tissue in an animal model.
Materials and Methods
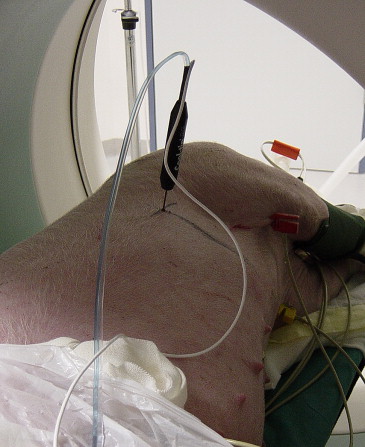
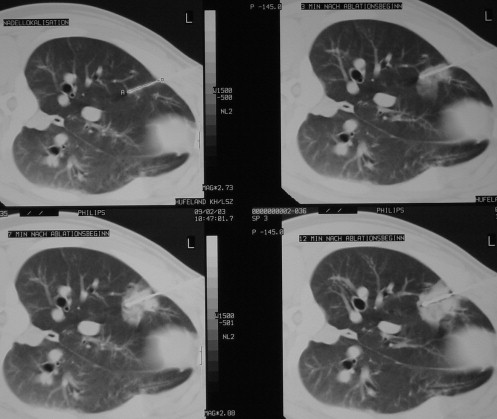
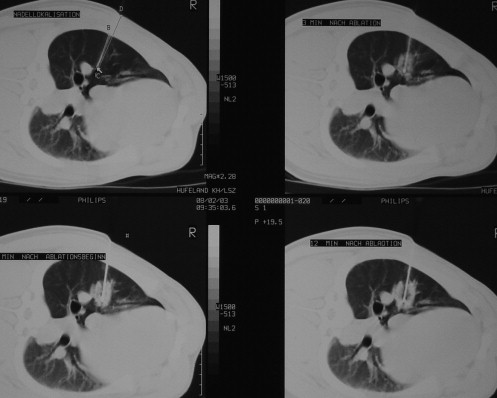
RF ablation of lung tissue was performed on eight swine under computed tomographic control. Group A ( n = 4) received peripheral ablation (subpleural needle placement) and group B ( n = 4) received central ablation (hilar needle placement). RF ablation was applied via a single 4.5-gauge internally cooled electrode with a 2-cm tip for 12 minutes. The ablation was monitored with computed tomography at 3, 7, and 12 minutes, and 10 minutes after ablation. After 3, 7, 40, and 60 days, computed tomography was performed, and the animals were sacrificed to examine the lung tissue both macroscopically and histopathologically.
Results
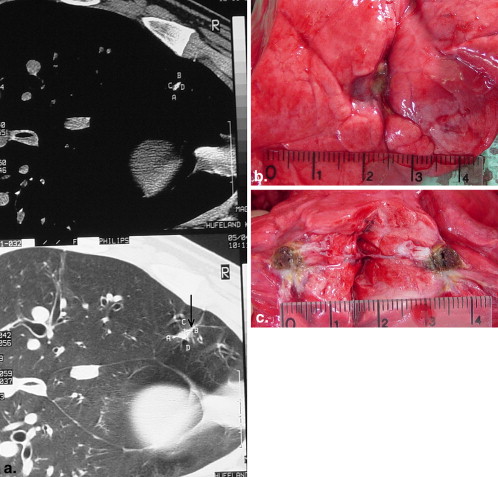
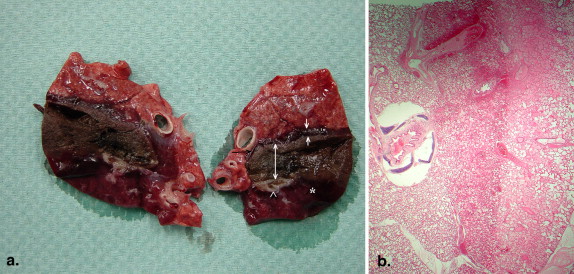
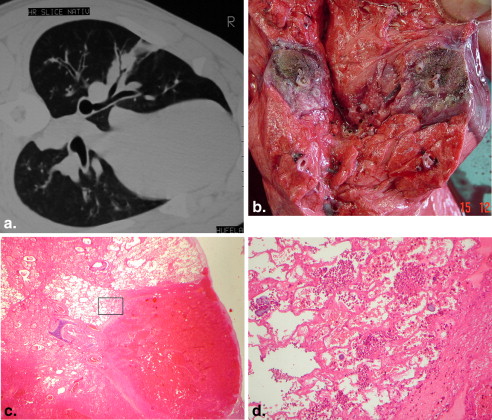
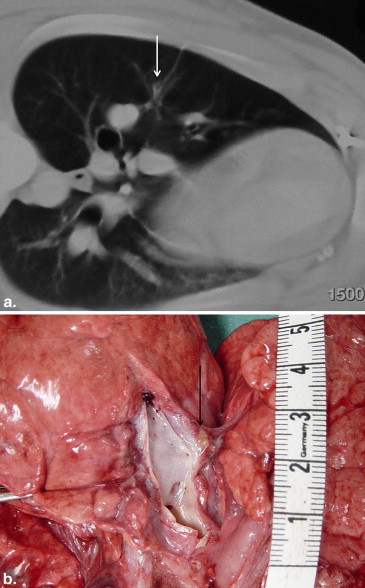
There were no deaths from RF ablation. In group A, coagulative necrosis was resorbed almost completely and transformed into a fibrotic scar after 60 days. No pneumothorax, pleural effusion, or lung abscess was observed. In group B, there was also a transformation of the necrosis into connective tissue. Neither the pulmonary vessels nor the bronchi of the hilum abutting the coagulative necrosis were damaged. After 60 days, no vascular thrombosis, bleeding, aneurysm, bronchial stenosis, or bronchopulmonary fistula was observed.
Conclusion
RF ablation of lung tissue affects coagulation necrosis, causing scar transformation. There was no damage to either great vessels or bronchi. The application of RF ablation for tumors located in or near functional structures appears feasible without severe complications.
Radiofrequency (RF) ablation is a therapeutic option for the treatment of unresectable lung tumors . To obtain complete necrosis of tumors, the extent of coagulative necrosis must include 0.5–1.0 cm of surrounding normal lung tissue . RF ablation has also been applied to central tumors near vital structures of the lung hilum. However, there are a few reports describing severe complications after ablation. Other authors applying ablation to hilar lesions did not observe such complications . There are no reports investigating the effects of thermal coagulation on either large vessels or bronchi. The pneumothorax rate was 28%, with a 10% chest tube insertion rate . To assess the risk of peripheral and central RF ablation, we believe it is necessary to investigate the effects of RF ablation on normal lung tissue, especially on the lung hilum, in an animal model.
Materials and methods
Experiments were carried out on eight 3- to 5-month-old female pigs (Deutsches Landschwein) with a mean weight of 34.3 kg (range, 29–43 kg). The animals were divided into two groups: group A consisted of four pigs that received peripheral RF ablation; group B consisted of four pigs that received central RF ablation. At 3, 7, 40, and 60 days after RF ablation, the pigs were examined with computed tomography (CT). The pigs in each group were sacrificed immediately after CT. Lung specimens were removed and prepared for macroscopic and histopathological examination. The present study conformed to the Guidelines for the Care and Use of Laboratory Animals (National Veterinary Protection Law, No. 30, 1998; Thuringia, Germany) and was approved by the Veterinary Department of the Thuringian State Authority for Food Protection and Fair Trading.
Anesthesia
Get Radiology Tree app to read full this article<
RF Ablation
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Evaluation
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Results
Get Radiology Tree app to read full this article<
Group A
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Group B
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Discussion
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Acknowledgments
Get Radiology Tree app to read full this article<
References
1. Dupuy D.E., Zagoria R.J., Akerley W., et. al.: Percutaneous radiofrequency ablation of malignancies in the lung. AJR Am J Roentgenol 2000; 174: pp. 57-59.
2. Steinke K., Habicht J.M., Thomsen S., et. al.: CT-guided radiofrequency ablation of a pulmonary metastasis followed by surgical resection. Cardiovasc Intervent Radiol 2002; 25: pp. 543-546.
3. Suh R.D., Wallace A.B., Sheehan R.E., et. al.: Unresectable pulmonary malignancies: CT-guided percutaneous radiofrequency ablation—preliminary results. Radiology 2003; 229: pp. 821-829.
4. Yasui K., Kanazawa S., Sano Y., et. al.: Thoracic tumors treated with CT-guided radiofrequency ablation: initial experience. Radiology 2004; 231: pp. 850-857.
5. Lee J.M., Jin G.Y., Goldberg S.N., et. al.: Percutaneous radiofrequency ablation for inoperable non-small cell lung cancer and metastases: preliminary report. Radiology 2004; 230: pp. 125-134.
6. Herrera L.J., Fernando H.C., Perry Y., et. al.: Radiofrequency ablation of pulmonary malignant tumors in nonsurgical candidates. J Thorac Cardiovasc Surg 2003; 125: pp. 929-937.
7. Vaughn C., Mychaskiw G., Sewell P.: Massive hemorrhage during radiofrequency ablation of a pulmonary neoplasm. Anesth Analg 2002; 94: pp. 1149-1151.
8. Linden P.A., Wee J.O., Jaklitsch M.T., et. al.: Extending indications for radiofrequency ablation of lung tumors through an intraoperative approach. Ann Thorac Surg 2008; 85: pp. 420-423.
9. Kelekis A.D., Thanos L., Mylona S., et. al.: Percutaneous radiofrequency ablation of lung tumors with expandable needle electrodes: current status. Eur Radiol 2006; 16: pp. 2471-2482.
10. Shu Yan Huo A., Lawson Morris D., King J., et. al.: Use of percutaneous radiofrequency ablation in pulmonary metastases from renal cell carcinoma. Ann Surg Oncol 2009; 16: pp. 3169-3175.
11. Simon C.J., Dupuy D.E., DiPetrillo T.A., et. al.: Pulmonary radiofrequency ablation: long-term safety and efficacy in 153 patients. Radiology 2007; 243: pp. 268-275.
12. Schneider T., Warth A., Herpel E., et. al.: Intraoperative radiofrequency ablation of lung metastases and histologic evaluation. Ann Thorac Surg 2009; 87: pp. 379-384.
13. Ambrogi M.C., Fontanini G., Cioni R., et. al.: Biologic effects of radiofrequency thermal ablation on non-small cell lung cancer: results of a pilot study. J Thorac Cardiovasc Surg 2006; 131: pp. 1002-1006.
14. Ahmed M., Liu Z., Afzal K.S., et. al.: Radiofrequency ablation: effect of surrounding tissue composition on coagulation necrosis in a canine tumor model. Radiology 2004; 230: pp. 761-767.
15. Yamamoto A., Nakamura K., Matsuoka T., et. al.: Radiofrequency ablation in a porcine lung model: correlation between CT and histopathologic findings. AJR Am J Roentgenol 2005; 185: pp. 1299-1306.