Purpose
To evaluate the accuracy of a novel combined electromagnetic (EM) navigation/image fusion system for biopsy of small lesions.
Materials and Methods
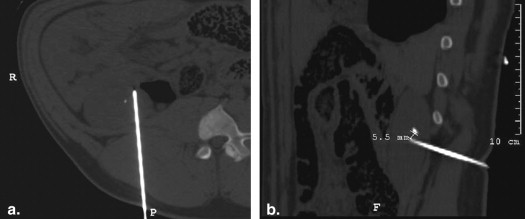
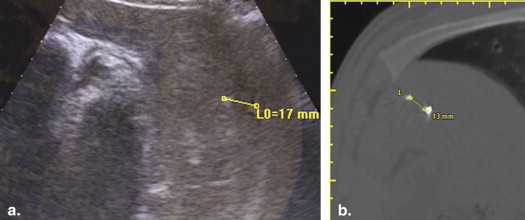
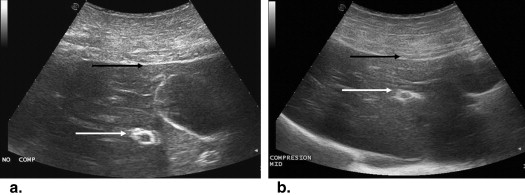
Using ultrasound (US) guidance, metallic (2 × 1 mm) targets were imbedded in the paraspinal muscle ( n = 28), kidney ( n = 18), and liver ( n = 4) of four 55- to 65-kg pigs. Baseline helical computed tomography (CT) imaging (Brilliance; Philips) identified these biopsy targets and six and nine cutaneous fiducial markers. CT data were imported into a MyLab Twice system (Esaote, Genoa, Italy) for CT/US image fusion. After verification of successful image fusion, baseline registration error and respiratory motion error were assessed by documenting deviation of the US and CT position of the targets in real time. Biopsy targeting was subsequently performed under conditions of normal respiratory using 15-cm 16G eTrax needles (Civco). To mimic the conditions of poor US visualization, only reconstructed CT information was displayed during biopsy. Accuracy of targeting was measured by repeat CT scanning as the distance of the needle tip to the target center. Targeting accuracy of free-hand vs. guided technique, and electromagnetic (EM) sensor positioning (ie, on the hub or within the needle stylus tip) were evaluated.
Results
In muscle, needle registration error was 0.9 ± 1.2 mm and respiratory motion error 4.0 ± 1.0 mm. Target accuracy was 4.0 ± 3.2 mm when an EM sensor was imbedded in the needle tip. Yet, with the EM sensor back on the needle hub, greater targeting accuracy was achieved using an US guide (3.2 ± 1.6 mm) vs. freehand (5.7 ± 3.2 mm, P = .04). For kidney, registration error was 1.8 ± 1.7 mm and respiratory motion error 4.9 ± 1.0 mm. For the deeper kidney targets, target accuracy was 4.4 ± 3.2 mm with a tip EM sensor, which was an improvement over the hub EM sensor positioning (9.3 ± 4.6 mm; P < .01). An additional source of fusion error was noted for liver. Beyond 17 ± 1 mm of respiratory motion, targets were observed to move >3 cm with US transducer/needle compression resulting in 14 ± 1.4 mm targeting accuracy.
Conclusions
A combined image-fusion/EM tracking platform can provide a high degree of needle placement accuracy (<5 mm) when targeting small lesions. Results fall within accuracy of respiratory error; with best results obtained by incorporating an EM sensor into the tip of the biopsy system.
Introduction
Precise device placement and positioning are the basis of every successful image-guided biopsy, tumor ablation, and most other interventional radiology procedures . Usually, this is accomplished using imaging guidance (computed tomography [CT], ultrasound [US], magnetic resonance imaging [MRI], or fluoroscopy), and depends on the operator’s ability and experience. Of these, US is often considered the ideal tool for daily practice in most solid organs because it enables real-time monitoring, is readily available, mobile, and lacks ionizing radiation. Yet, use of this modality is not always possible because of limitations in visualization of the target or surrounding critical structures . An experienced sonographer can often overcome less than ideal visualization at US by incorporating mental registration of the anatomic information from offline modalities (such as contrast-enhanced CT, MRI, and positron emission tomography-CT). Yet, as procedures get more complex, synthesis of all this requisite information becomes more challenging. This in turn has spawned the development of various devices/algorithms to assist in real-time, on-screen image fusion .
An additional approach to address this issue is navigation, electromagnetic (EM) tracking, can potentially provide spatial navigation information in real-time based on reconstruction of, and interaction with, datasets of previously acquired images . This can enable therapeutic and monitoring devices to be accurately positioned with use of an EM navigation system alone, without the need to continually perform imaging of the patient.
Get Radiology Tree app to read full this article<
Materials and methods
Get Radiology Tree app to read full this article<
Experimental Study Overview
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Animal Model
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
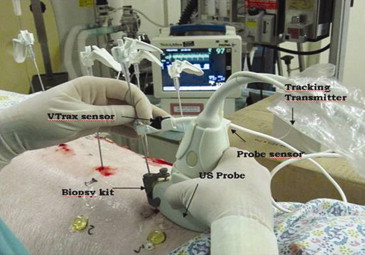
Biopsy Navigation System
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
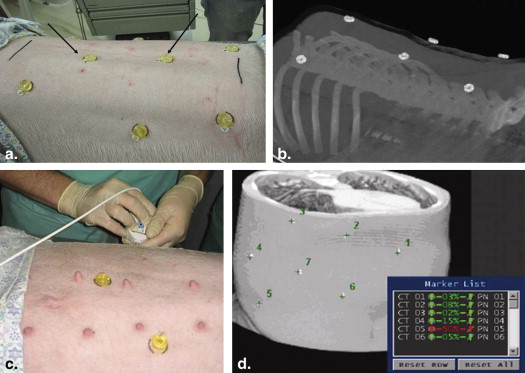
CT Data Acquisition
Get Radiology Tree app to read full this article<
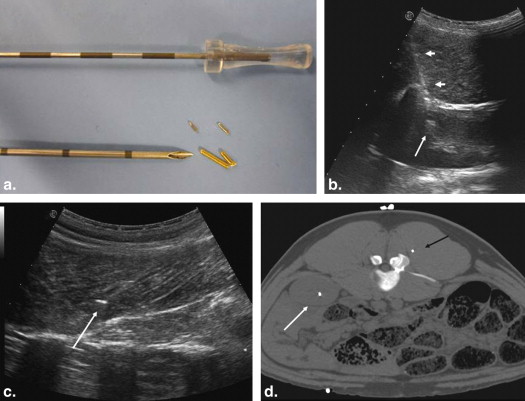
Biopsy Methodology
Get Radiology Tree app to read full this article<
Study Procedure
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Study Endpoints
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Statistical Analysis
Get Radiology Tree app to read full this article<
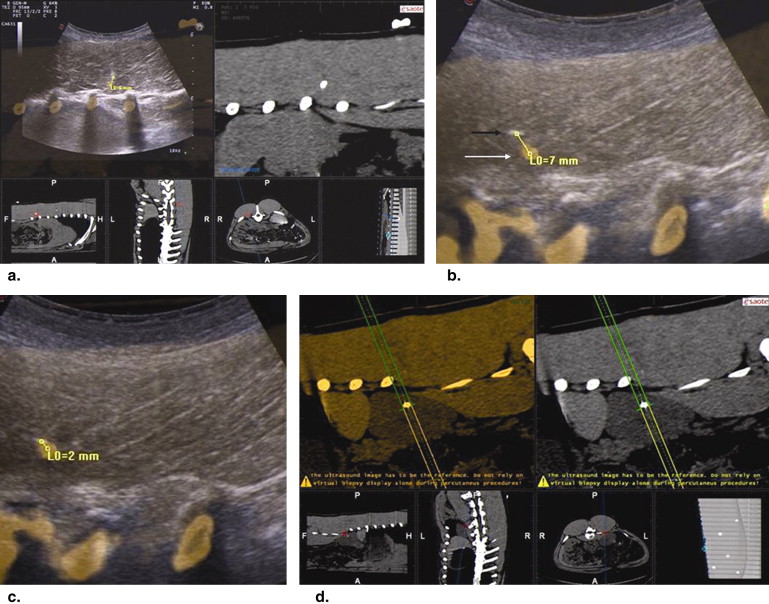
Results
Get Radiology Tree app to read full this article<
Paraspinal Muscle
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Kidney
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Liver
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Discussion
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
References
1. Goldberg S.N., Grassi C.J., Cardella J.F., et. al.: Image-guided tumor ablation: standardization of terminology and reporting criteria. Society of Interventional Radiology Technology Assessment Committee; International Working Group on Image-Guided Tumor Ablation. Radiology 2005; 235: pp. 728-739.
2. Appelbaum L., Sosna J., Nissenbaum Y., et. al.: Electromagnetic navigation system for CT-guided biopsy of small lesions. AJR Am J Roentgenol 2011; 196: pp. 1194-2000.
3. Iagnocco A., Perella C., D’Agostino M.A., et. al.: Magnetic resonance and ultrasonography real-time fusion imaging of the hand and wrist in osteoarthritis and rheumatoid arthritis. Rheumatology (Oxford) 2011; 50: pp. 1409-1413.
4. Yu X., Liu F., Liang P., et. al.: Microwave ablation assisted by a computerised tomography-ultrasonography fusion imaging system for liver lesions: an ex vivo experimental study. Int J Hypertherm 2011; 27: pp. 172-179.
5. Krücker J., Xu S., Venkatesan A., et. al.: Clinical utility of real-time fusion guidance for biopsy and ablation. J Vasc Interv Radiol 2011; 22: pp. 515-524.
6. Wood B.J., Zhang H., Durrani A., et. al.: Navigation with electromagnetic tracking for interventional radiology procedures: a feasibility study. J Vasc Interv Radiol 2005; 16: pp. 493-505.
7. Seiler P.G., Blattman H., Kirsch R.K., et. al.: A novel tracking technique for the continuous precise measurement of tumour positions in conformal radiotherapy. Phys Med Biol 2000; 45: pp. N103-N110.
8. Frantz D.D., Wiles A.D., Leis S.E., et. al.: Accuracy assessment protocols for electromagnetic tracking systems. Phys Med Biol 2003; 48: pp. 2241-2251.
9. Solomon S.B., White P., Wiener C.M., et. al.: Three-dimensional CT-guided bronchoscopy with a real time electromagnetic position sensor. Chest 2000; 118: pp. 1783-1787.
10. Solomon S.B., Dickfield T., Calkins H.: Real-time cardiac catheter navigation on three-dimensional CT images. J Intervent Cardiac Electrophysiol 2003; 8: pp. 27-36.
11. Solomon S.B., Magee C.A., Acker D.E., et. al.: Experimental nonfluoroscopic placement of inferior vena cava filters: use of an electromagnetic navigation system with previous CT data. J Vasc Intervent Radiol 1999; 10: pp. 92-95.
12. Solomon S.B., Magee C., Acker D.E., et. al.: TIPS placement in swine, guided by electromagnetic real-time needle tip localization displayed on previously acquired 3-D CT. Cardiovasc Intervent Radiol 1999; 22: pp. 411-414.
13. Pinto P.A., Chung P.H., Rastinehad A.R., et. al.: Magnetic resonance imaging/ultrasound fusion guided prostate biopsy improves cancer detection following transrectal ultrasound biopsy and correlates with multiparametric magnetic resonance imaging. J Urol 2011; 186: pp. 1281-1285.
14. Banovac F., Cheng P., Campos-Nanez E., et. al.: Radiofrequency ablation of lung tumors in swine assisted by a navigation device with preprocedural volumetric planning. J Vasc Interv Radiol 2010; 21: pp. 122-129.
15. Buckner C.A., Venkatesan A., Locklin J.K., et. al.: Real-time sonography with electromagnetic tracking navigation for biopsy of a hepatic neoplasm seen only on arterial phase computed tomography. J Ultrasound Med 2011; 30: pp. 253-256.
16. Venkatesan A.M., Kadoury S., Abi-Jaoudeh N., et. al.: Real-time FDG PET guidance during biopsies and radiofrequency ablation using multimodality fusion with electromagnetic navigation. Radiology 2011; 260: pp. 848-856.
17. Mueller P.R., vanSonnenberg E.: Interventional radiology in the chest and abdomen. N Engl J Med 1990; 322: pp. 1364-1374.
18. Liu F.Y., Yu X.L., Liang P., et. al.: Microwave ablation assisted by a real-time virtual navigation system for hepatocellular carcinoma undetectable by conventional ultrasonography. Eur J Radiol 2012; 81: pp. 1455-1459.
19. Minami Y., Kitai S., Kudo M.: Treatment response assessment of radiofrequency ablation for hepatocellular carcinoma: usefulness of virtual CT sonography with magnetic navigation. Eur J Radiol 2012; 81: pp. e277-e280.
20. Nakai M., Sato M., Sahara S., et. al.: Radiofrequency ablation assisted by real-time virtual sonography and CT for hepatocellular carcinoma undetectable by conventional sonography. Cardiovasc Intervent Radiol 2009; 32: pp. 62-69.
21. Krucker J., Xu S., Glossop N., et. al.: Electromagnetic tracking for thermal ablation and biopsy guidance: clinical evaluation of spatial accuracy. J Vasc Interv Radiol 2007; 18: pp. 1141-1150.
22. Wood B.J., Zhang H., Durrani A., et. al.: Navigation with electromagnetic tracking for interventional radiology procedures: a feasibility study. J Vasc Interv Radiol 2005; 16: pp. 493-505.
23. Santos R.S., Gupta A., Ebright M.I., et. al.: Electromagnetic navigation to aid radiofrequency ablation and biopsy of lung tumors. Ann Thorac Surg 2010; 89: pp. 265-268.
24. Timmerman R.D., Park C., Kavanagh B.D.: The North American experience with stereotactic body radiation therapy in non-small cell lung cancer. J Thorac Oncol 2007; 2: pp. S101-S112.