Rationale and Objectives
Resting-state (RS) networks, revealed by functional magnetic resonance imaging (fMRI) studies in healthy volunteers, have never been evaluated in anesthetized patients with brain tumors. Our purpose was to examine the presence of residual brain activity on the auditory network during propofol-induced loss of consciousness in patients with brain tumors.
Materials and Methods
Twenty subjects with intracranial masses were prospectively studied by means of intraoperative RS-fMRI acquisitions before any craniectomy. After performing single-subject independent component analysis, spatial maps and time courses were assigned to an auditory RS network template from the literature and compared via spatial regression coefficients.
Results
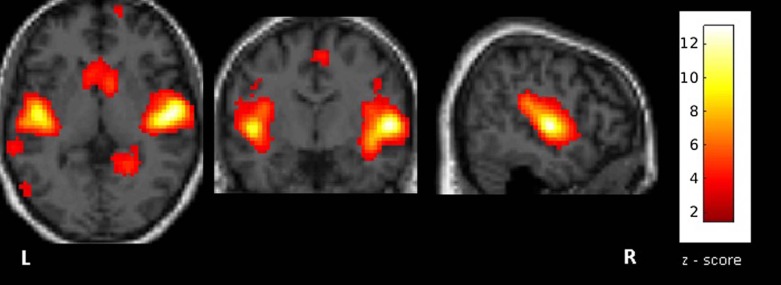
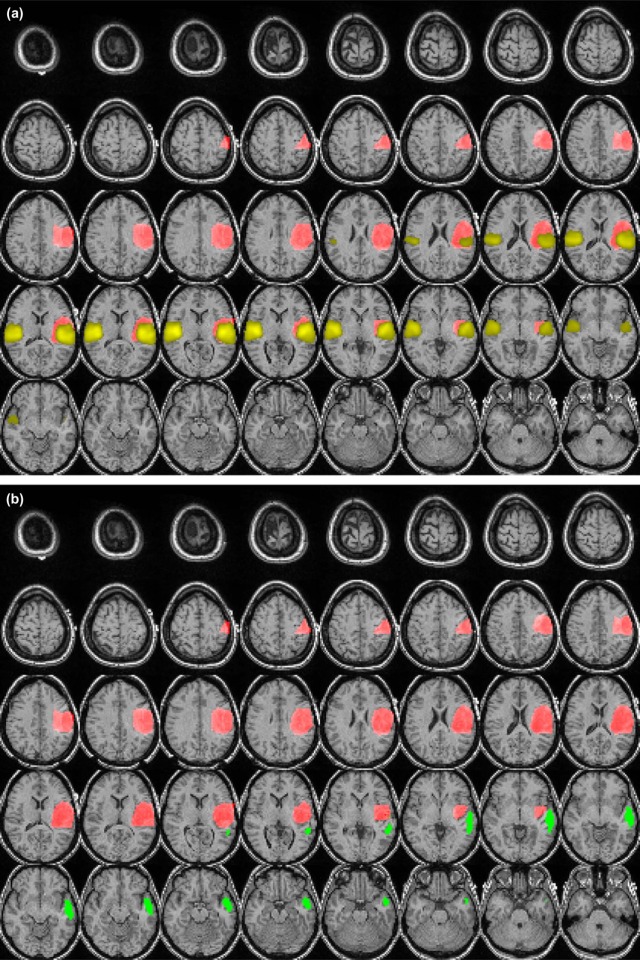
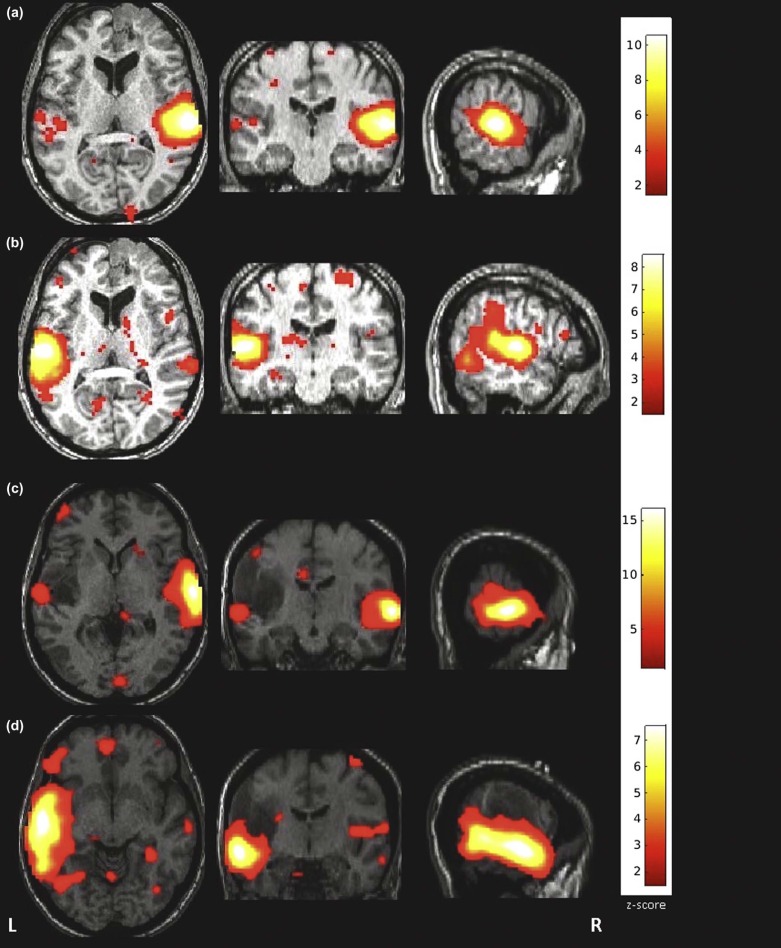
All fMRI data were of sufficient quality for further postprocessing. In all but two patients, the RS functional activity of the auditory network could be successfully mapped. In almost all patients, contralateral activation of the auditory network was present. No significant difference was found between the mean distance of the RS activity clusters and the lesion periphery for tumors located in the temporal gyri vs. those in other brain regions. The spatial deviation between the activated cluster in our experiment and the template was significantly ( P = 0.04) higher in patients with tumors located in the temporal gyri than in patients with tumors located in other regions.
Conclusions
Propofol-induced anesthesia in patients with intracranial lesions does not alter the blood-oxygenation level-depended signal, and independent component analysis of intraoperative RS-fMRI may allow assessment of the auditory network in a clinical setting.
Introduction
Resting-state functional magnetic resonance imaging (RS-fMRI) acquisitions, based on system-wide coherent blood-oxygenation level dependent (BOLD) signal fluctuations, visualize a set of regions suggesting an underlying functional relationship despite deprivation of external stimuli . In humans, fMRI-visualized RS activity has been shown to be stable throughout light sleep/wake cycles , during light sleep, deep sleep, or sedation , as well as during different levels of consciousness including propofol-induced loss of consciousness . The partial preservation of functional connectivity in the absence of consciousness has been attributed to preserved anatomical connections dissociated from higher cognitive functions .
Thus far, however, these RS networks, revealed by fMRI studies mostly in healthy volunteers, have never been evaluated in anesthetized patients with brain tumors. The present study investigated the feasibility of acquiring whole-brain RS-fMRI measurements in patients with brain tumors by means of intraoperative fMRI during pharmacological modulation of the level of consciousness. The anesthetic drug used was propofol because of its advantage over isoflurane/sevoflurane in keeping low subdural intracranial pressure and arteriovenous oxygen difference while keeping higher cerebral perfusion pressure in patients with brain tumors . Under the proof-of-principle study design, we orientated in the RS activity of the auditory cortex because it has been shown in many studies to be robustly identifiable across healthy subjects and patients , its analysis is relatively simple compared to other networks , and previous works have tested the influence of different anesthetics on its activation in healthy subjects. The auditory network involves the superior temporal gyrus, the transverse temporal gyrus (Heschl’s gyrus), the insula, and the postcentral gyrus. The primary auditory cortex has also known interactions with the angular gyrus, the supramarginal gyrus, Broca’s, and Wernicke’s areas.
Get Radiology Tree app to read full this article<
Materials and Methods
Ethics Statement
Get Radiology Tree app to read full this article<
Subjects
Get Radiology Tree app to read full this article<
Intraoperative MRI
Get Radiology Tree app to read full this article<
Anesthesia Protocol
Get Radiology Tree app to read full this article<
Image Processing and Data Analysis
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Table 1
Demographics, Clinical Data, and Resting-state BOLD Fluctuations Analysis of the Auditory Network in Our Patient Cohort
Patient (Age/Sex) Tumor Site (Side) z-value Ratio Tumor-Cluster Distance (mm) Template-Cluster Distance (mm) 1 (55/M) Gyrus temporalis medius (R) 13.9 3.9 60.4 15.0 2 (39/F) Gyrus temporalis medius et superior (L) 15.8 4.0 55.2 12.6 3 (23/M) Gyrus temporalis (L) 11.1 1.7 50.5 11.0 4 (27/M) Gyrus temporalis inferior (R) 13.1 7.3 43.9 16.5 5 (19/F) Temporomesial and temporobasal (R) 1.9 2.6 46.5 26.8 6 (33/M) Gyrus temporalis medius et superior (R) 12.0 1.9 29.7 12.8 7 (27/M) Skull base (R) 9.5 3.4 n.a. \* n.a. \* 8 (46/F) Thalamus (R) 3.7 4.2 48.4 13.6 9 (52/F) Skull base (L) 10.3 2.3 n.a. \* n.a. \* 10 (44/M) Gyrus parahippocampalis (L) 9.2 4.9 24.3 12.0 11 (34/M) Gyrus parahippocampalis (L) 14.4 3.2 39.9 14.7 12 (77/F) Gyrus frontalis superior (L) 12.2 0.9 41.2 8.7 13 (30/M) Gyrus frontalis inferior et insula (L) 8.8 6.9 52.8 3.5 14 (30/F) Gyrus frontalis superior et medius (R) 8.6 3.4 18.0 6.3 15 (72/M) Gyrus postcentralis (L) 10.5 2.3 34.1 12.3 16 (48/F) Gyrus postcentralis (L) 9.8 1.8 52.6 12.8 17 (52/F) Gyrus postcentralis (L) 13.0 2.9 60.2 15.7 18 (24/M) Gyrus precentralis (R) 9.1 2.3 32.1 8.2 19 (25/M) Gyrus precentralis (L) 3.2 3.3 60.9 14.1 20 (29/F) Gyrus precentralis (L) 9.6 1.8 53.8 18.5
BOLD, blood-oxygenation level dependent.
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Results
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Discussion
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Conclusions
Get Radiology Tree app to read full this article<
References
1. Gusnard D.A., Raichle M.E., Raichle M.E.: Searching for a baseline: functional imaging and the resting human brain. Nat Rev Neurosci 2001; 2: pp. 685-694.
2. Larson-Prior L.J., Power J.D., Vincent J.L., et. al.: Modulation of the brain’s functional network architecture in the transition from wake to sleep. Prog Brain Res 2011; 193: pp. 277-294.
3. Fukunaga M., Horovitz S.G., van Gelderen P., et. al.: Large-amplitude, spatially correlated fluctuations in BOLD fMRI signals during extended rest and early sleep stages. Magn Reson Imaging 2006; 24: pp. 979-992.
4. Horovitz S.G., Fukunaga M., de Zwart J.A., et. al.: Low frequency BOLD fluctuations during resting wakefulness and light sleep: a simultaneous EEG-fMRI study. Hum Brain Mapp 2008; 29: pp. 671-682.
5. Greicius M.D., Kiviniemi V., Tervonen O., et. al.: Persistent default-mode network connectivity during light sedation. Hum Brain Mapp 2008; 29: pp. 839-847.
6. Horovitz S.G., Braun A.R., Carr W.S., et. al.: Decoupling of the brain’s default mode network during deep sleep. Proc Natl Acad Sci U S A 2009; 106: pp. 11376-11381.
7. Boveroux P., Vanhaudenhuyse A., Bruno M.A., et. al.: Breakdown of within- and between-network resting state functional magnetic resonance imaging connectivity during propofol-induced loss of consciousness. Anesthesiology 2010; 113: pp. 1038-1053.
8. Peltier S.J., Kerssens C., Hamann S.B., et. al.: Functional connectivity changes with concentration of sevoflurane anesthesia. Neuroreport 2005; 16: pp. 285-288.
9. Vanhaudenhuyse A., Noirhomme Q., Tshibanda L.J., et. al.: Default network connectivity reflects the level of consciousness in non-communicative brain-damaged patients. Brain 2010; 133: pp. 161-171.
10. Boly M., Tshibanda L., Vanhaudenhuyse A., et. al.: Functional connectivity in the default network during resting state is preserved in a vegetative but not in a brain dead patient. Hum Brain Mapp 2009; 30: pp. 2393-2400.
11. Petersen K.D., Landsfeldt U., Cold G.E., et. al.: Intracranial pressure and cerebral hemodynamic in patients with cerebral tumors: a randomized prospective study of patients subjected to craniotomy in propofol-fentanyl, isoflurane-fentanyl, or sevoflurane-fentanyl anesthesia. Anesthesiology 2003; 98: pp. 329-336.
12. Damoiseaux J.S., Rombouts S.A., Barkhof F., et. al.: Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci U S A 2006; 103: pp. 13848-13853.
13. Maudoux A., Lefebvre P., Cabay J.E., et. al.: Auditory resting-state network connectivity in tinnitus: a functional MRI study. PLoS ONE 2012; 7: pp. e36222.
14. Allen E.A., Erhardt E.B., Damaraju E., et. al.: A baseline for the multivariate comparison of resting-state networks. Front Syst Neurosci 2011; 5: pp. 2.
15. Kerssens C., Hamann S., Peltier S., et. al.: Attenuated brain response to auditory word stimulation with sevoflurane: a functional magnetic resonance imaging study in humans. Anesthesiology 2005; 103: pp. 11-19.
16. Veselis R.A., Feshchenko V.A., Reinsel R.A., et. al.: Propofol and thiopental do not interfere with regional cerebral blood flow response at sedative concentrations. Anesthesiology 2005; 102: pp. 26-34.
17. Chen X., Xu B.N., Meng X., et. al.: Dual-room 1.5-T intraoperative magnetic resonance imaging suite with a movable magnet: implementation and preliminary experience. Neurosurg Rev 2012; 35: pp. 95-109. discussion 109-110
18. Bell A.J., Sejnowski T.J.: An information-maximization approach to blind separation and blind deconvolution. Neural Comput 1995; 7: pp. 1129-1159.
19. Holodny A.I., Schulder M., Liu W.C., et. al.: The effect of brain tumors on BOLD functional MR imaging activation in the adjacent motor cortex: implications for image-guided neurosurgery. AJNR Am J Neuroradiol 2000; 21: pp. 1415-1422.
20. Kokkonen S.M., Kiviniemi V., Makiranta M., et. al.: Effect of brain surgery on auditory and motor cortex activation: a preliminary functional magnetic resonance imaging study. Neurosurgery 2005; 57: pp. 249-256. discussion 249–256
21. Kokkonen S.M., Nikkinen J., Remes J., et. al.: Preoperative localization of the sensorimotor area using independent component analysis of resting-state fMRI. Magn Reson Imaging 2009; 27: pp. 733-740.
22. Zhang D., Johnston J.M., Fox M.D., et. al.: Preoperative sensorimotor mapping in brain tumor patients using spontaneous fluctuations in neuronal activity imaged with functional magnetic resonance imaging: initial experience. Neurosurgery 2009; 65: pp. 226-236.
23. Johnston A.J., Steiner L.A., Chatfield D.A., et. al.: Effects of propofol on cerebral oxygenation and metabolism after head injury. Br J Anaesth 2003; 91: pp. 781-786.
24. Heinke W., Fiebach C.J., Schwarzbauer C., et. al.: Sequential effects of propofol on functional brain activation induced by auditory language processing: an event-related functional magnetic resonance imaging study. Br J Anaesth 2004; 92: pp. 641-650.
25. Khalili-Mahani N., van Osch M.J., de Rooij M.D., et. al.: Spatial heterogeneity of the relation between resting-state connectivity and blood flow: an important consideration for pharmacological studies. Hum Brain Mapp 2014; 35: pp. 929-942.
26. Hou B.L., Bradbury M., Peck K.K., et. al.: Effect of brain tumor neovasculature defined by rCBV on BOLD fMRI activation volume in the primary motor cortex. Neuroimage 2006; 32: pp. 489-497.
27. Zou Q., Wu C.W., Stein E.A., et. al.: Static and dynamic characteristics of cerebral blood flow during the resting state. Neuroimage 2009; 48: pp. 515-524.
28. Qiu M., Ramani R., Swetye M., et. al.: Anesthetic effects on regional CBF, BOLD, and the coupling between task-induced changes in CBF and BOLD: an fMRI study in normal human subjects. Magn Reson Med 2008; 60: pp. 987-996.
29. Quigley M., Cordes D., Wendt G., et. al.: Effect of focal and nonfocal cerebral lesions on functional connectivity studied with MR imaging. AJNR Am J Neuroradiol 2001; 22: pp. 294-300.
30. Fukunaga M., Horovitz S.G., de Zwart J.A., et. al.: Metabolic origin of BOLD signal fluctuations in the absence of stimuli. J Cereb Blood Flow Metab 2008; 28: pp. 1377-1387.
31. Shulman R.G., Rothman D.L., Hyder F.: Stimulated changes in localized cerebral energy consumption under anesthesia. Proc Natl Acad Sci U S A 1999; 96: pp. 3245-3250.
32. Feldman S.C., Chu D., Schulder M., et. al.: The blood oxygen level-dependent functional MR imaging signal can be used to identify brain tumors and distinguish them from normal tissue. AJNR Am J Neuroradiol 2009; 30: pp. 389-395.
33. Dagli M.S., Ingeholm J.E., Haxby J.V.: Localization of cardiac-induced signal change in fMRI. Neuroimage 1999; 9: pp. 407-415.
34. van de Ven V.G., Formisano E., Prvulovic D., et. al.: Functional connectivity as revealed by spatial independent component analysis of fMRI measurements during rest. Hum Brain Mapp 2004; 22: pp. 165-178.
35. Kollndorfer K., Fischmeister F.P., Kasprian G., et. al.: A systematic investigation of the invariance of resting-state network patterns: is resting-state fMRI ready for pre-surgical planning?. Front Hum Neurosci 2013; 7: pp. 95.
36. Tie Y., Rigolo L., Norton I.H., et. al.: Defining language networks from resting-state fMRI for surgical planning—a feasibility study. Hum Brain Mapp 2014; 35: pp. 1018-1030.
37. Ma L., Wang B., Chen X., et. al.: Detecting functional connectivity in the resting brain: a comparison between ICA and CCA. Magn Reson Imaging 2007; 25: pp. 47-56.
38. van den Heuvel M.P., Hulshoff Pol H.E.: Exploring the brain network: a review on resting-state fMRI functional connectivity. Eur Neuropsychopharmacol 2010; 20: pp. 519-534.
39. Cole D.M., Smith S.M., Beckmann C.F.: Advances and pitfalls in the analysis and interpretation of resting-state FMRI data. Front Syst Neurosci 2010; 4: pp. 8.
40. Soddu A., Vanhaudenhuyse A., Bahri M.A., et. al.: Identifying the default-mode component in spatial IC analyses of patients with disorders of consciousness. Hum Brain Mapp 2012; 33: pp. 778-796.