Rationale and Objectives
Recent reports suggest that cancer cells may use glutamine, instead of glucose, as an alternative source of metabolic energy. This suggests that hyperpolarized 13 C glutamine may be useful as a magnetic resonance spectroscopy (MRS) imaging agent for detecting changes in glutamine metabolism in cancerous cells or tissues.
Materials and Methods
Synthesis of [5- 13 C-4- 2 H 2 ]-L-glutamine was accomplished through a seven-step synthetic pathway with a 44% overall yield. The introduction of two stable isotopes was performed by a NaB 2 H 4 -mixed anhydride reduction and K 13 CN-nuclophilic substitution, respectively. The desired [5- 13 C-4- 2 H 2 ]-L-glutamine was successfully obtained by a one-pot reaction of deprotection and controlled cyanide hydrolysis. Hyperpolarized [5- 13 C-4- 2 H 2 ]-L-glutamine samples were tested in human glioma cells (myc upregulated glia cells, SF188-Bcl-x L ). MRS signals were obtained with a 9.4 Tesla 89-mm bore nuclear magnetic resonance spectrometer and a direct-detection multi-nuclear probe.
Results
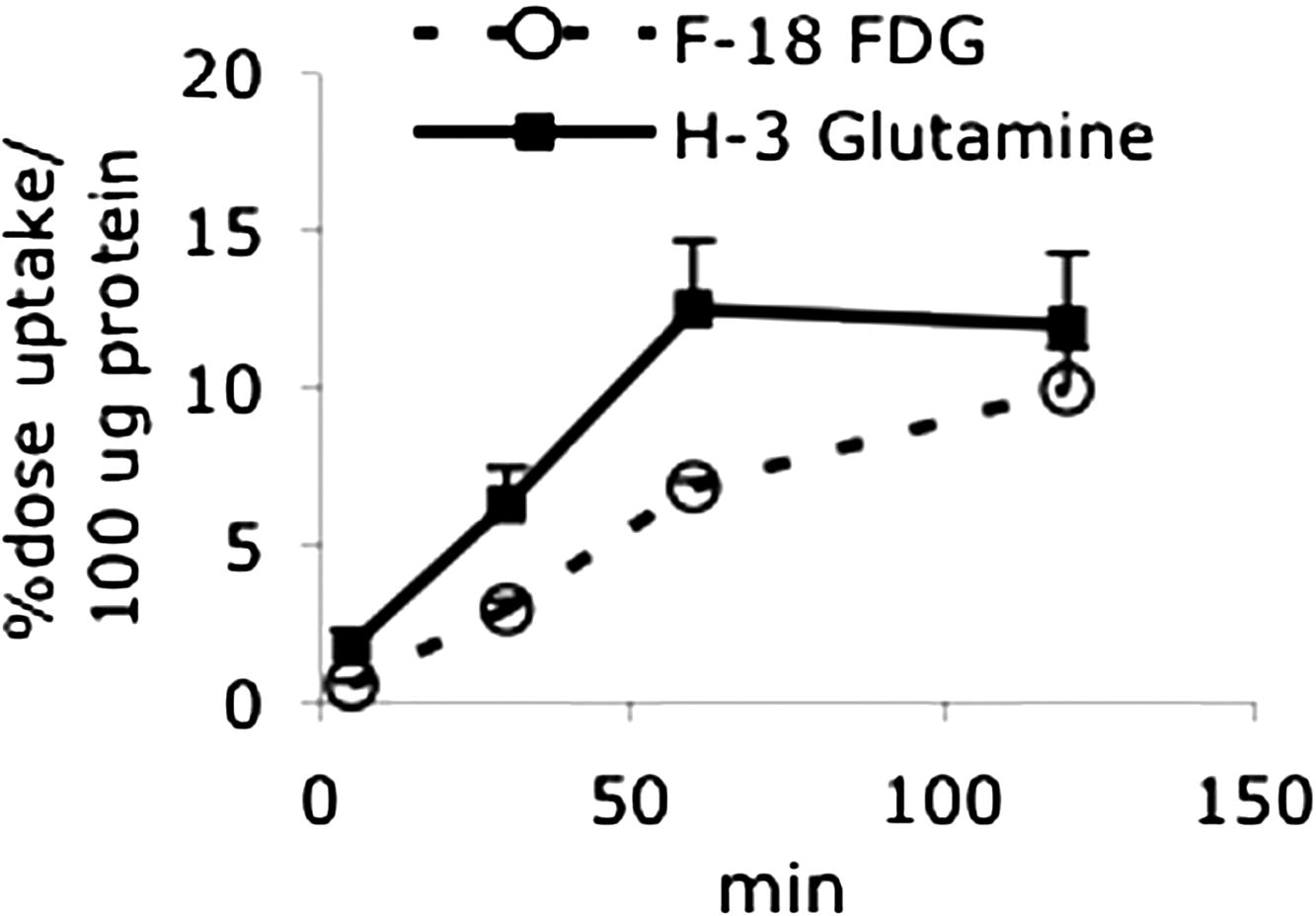
The initial degree of polarization for [5- 13 C-4- 2 H 2 ]-L-glutamine was ∼5% and the initial 13 C signal to noise ratio was ∼100:1. Glutamate was detected within seconds after the injection of hyperpolarized glutamine into the cells. The ratio of glutamate to glutamine was very high, indicating rapid conversion to glutamate. Similar cell uptake studies using [ 3 H]-L-glutamine also demonstrated cell uptakes higher than that of [ 18 F]fluorodeoxyglucose.
Conclusion
We are reporting the first example of using specifically deuterated [5- 13 C-4- 2 H 2 ]-L-glutamine in conjunction with hyperpolarized MRS for studying “glutaminolysis” in proliferating tumor cells.
It is well-known that cancer cells actively consume glucose as their energy source under aerobic conditions leading to the formation of lactate. This is referred to as the “Warburg effect” . This increased aerobic glycolysis in major tumor types is the foundation for using [ 18 F]fluorodeoxyglucose-positron emission tomography (FDG-PET) imaging as a tool in diagnosing cancer . Yet, some malignant tumors show ostensibly negative FDG-PET scans and cannot be reliably detected with FDG-PET. For example, primary tumors of the prostate that have not metastasized are generally not enhanced by FDG . In addition, FDG-PET only provides information about glucose uptake (Glut) and hexokinase levels. Inflammation interferes with interpretation because it will show up on the scan as a false positive. Recently, a series of articles have suggested that the FDG-negative tumors may use a different metabolic pathway, dubbed “glutaminolysis” . The results, at least partially, provide a probable explanation for the observation that FDG-PET sometimes fails to spot tumors in cancer patients. Recent reports have indicated that glutamine utilization (glutaminolysis) can be linked to upregulations of the oncogene, myc . Normally, upregulation of the oncogene, myc, as part of PI3K/Akt/mTOR signal pathway, stimulates glucose uptake and utilization through an aerobic glycolysis pathway leading to the production of lactic acid. However, during starvation or other stressed situations, cancer cells will switch their metabolic energy source from glucose to amino acids, such as glutamine, and other more abundant metabolites .
There is a high concentration of glutamine (0.5–1.0 mM) in the blood circulation and it has the highest concentration among all amino acids. Glutamine is a highly active molecule that can provide a source of nitrogen for various building blocks in the cells and can also be used as a source of energy. Actively proliferating tumors with a significant upregulation of myc gene can use glutamine to produce ATP as well as NADPH and the excess lactate . The major difference between aerobic glycolysis and glutaminolysis is that the latter needs functioning mitochondria and an active tricarboxylic acid (TCA) cycle. Glutamine on entering the cell releases a molecule of ammonia from the amide group and then glutamine transaminase strips off the second molecule of ammonia. Alpha-ketoglutarate is the entrance point to the TCA cycle for this metabolic pathway for energy production. To date, regulation of glutaminolysis has not been examined extensively. In a preliminary study, the oncogene myc was found to transcriptionally regulate glutamine uptake and glutaminase, which converts glutamine to glutamate . It has also been found that cancers with high levels of myc are glutamine addicted and undergo apoptosis when deprived of this amino acid .
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Results and discussion
Chemical Synthesis
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Hyperpolarization
Get Radiology Tree app to read full this article<
Cell Uptake Study by MR Spectroscopy
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Materials and methods
Synthetic Chemistry
Get Radiology Tree app to read full this article<
Hyperpolarization
Get Radiology Tree app to read full this article<
Cell Uptake Studies by MRS
Get Radiology Tree app to read full this article<
Dual-isotope Cell Uptake Study
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
References
1. Pedersen P.L.: Warburg, me and Hexokinase 2: multiple discoveries of key molecular events underlying one of cancers’ most common phenotypes, the “Warburg Effect”, i.e., elevated glycolysis in the presence of oxygen. J Bioenerg Biomembr 2007; 39: pp. 211-222.
2. Busk M., Horsman M.R., Kristjansen P.E., et. al.: Aerobic glycolysis in cancers: implications for the usability of oxygen-responsive genes and fluorodeoxyglucose-PET as markers of tissue hypoxia. Int J Cancer 2008; 122: pp. 2726-2734.
3. Robey I.F., Stephen R.M., Brown K.S., et. al.: Regulation of the Warburg effect in early-passage breast cancer cells. Neoplasia 2008; 10: pp. 745-756.
4. Thompson C.B.: Metabolic enzymes as oncogenes or tumor suppressors. N Engl J Med 2009; 360: pp. 813-815.
5. Gillies R.J., Robey I., Gatenby R.A.: Causes and consequences of increased glucose metabolism of cancers. J Nucl Med 2008; 49: pp. 24S-42S.
6. Ganapathy V., Thangaraju M., Prasad P.D.: Nutrient transporters in cancer: Relevance to Warburg hypothesis and beyond. Pharmacol Ther 2009; 121: pp. 29-40.
7. Turkbey B., Kobayashi H., Ogawa M., et. al.: Imaging of tumor angiogenesis: functional or targeted?. AJR Am J Roentgenol 2009; 193: pp. 304-313.
8. Wise D.R., DeBerardinis R.J., Mancuso A., et. al.: Myc regulates a transcriptional program that stimulates mitochondrial glutaminolysis and leads to glutamine addiction. Proc Natl Acad Sci U S A 2008; 105: pp. 18782-18787.
9. Deberardinis R.J., Sayed N., Ditsworth D., et. al.: Brick by brick: metabolism and tumor cell growth. Curr Opin Genet Dev 2008; 18: pp. 54-61.
10. DeBerardinis R.J., Lum J.J., Hatzivassiliou G., et. al.: The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab 2008; 7: pp. 11-20.
11. DeBerardinis R.J., Mancuso A., Daikhin E., et. al.: Beyond aerobic glycolysis: transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc Natl Acad Sci U S A 2007; 104: pp. 19345-19350.
12. Dang C.V., Kim J.W., Gao P., et. al.: The interplay between MYC and HIF in cancer. Nat Rev Cancer 2008; 8: pp. 51-56.
13. Yuneva M.: Finding an “Achilles’ heel” of cancer: the role of glucose and glutamine metabolism in the survival of transformed cells. Cell Cycle 2008; 7: pp. 2083-2089.
14. Gallagher F.A., Kettunen M.I., Day S.E., et. al.: 13C MR spectroscopy measurements of glutaminase activity in human hepatocellular carcinoma cells using hyperpolarized 13C-labeled glutamine. Magn Reson Med 2008; 60: pp. 253-257.
15. Chang T.C., Yu D., Lee Y.S., et. al.: Widespread microRNA repression by Myc contributes to tumorigenesis. Nat Genet 2008; 40: pp. 43-50.
16. Yuneva M., Zamboni N., Oefner P., et. al.: Deficiency in glutamine but not glucose induces MYC-dependent apoptosis in human cells. J Cell Biol 2007; 178: pp. 93-105.
17. Golman K., Petersson J.S.: Metabolic imaging and other applications of hyperpolarized 13C1. Acad Radiol 2006; 13: pp. 932-942.
18. Mancuso A., Zhu A., Beardsley N.J., et. al.: Artificial tumor model suitable for monitoring 31P and 13C NMR spectroscopic changes during chemotherapy-induced apoptosis in human glioma cells. Magn Reson Med 2005; 54: pp. 67-78.
19. Kohler S.J., Yen Y., Wolber J., et. al.: In vivo 13 carbon metabolic imaging at 3T with hyperpolarized 13C-1-pyruvate. Magn Reson Med 2007; 58: pp. 65-69.
20. Golman K., Ardenkjaer-Larsen J.H., Petersson J.S., et. al.: Molecular imaging with endogenous substances. Proc Natl Acad Sci U S A 2003; 100: pp. 10435-10439.
21. Golman K., Olsson L.E., Axelsson O., et. al.: Molecular imaging using hyperpolarized 13C. Br J Radiol 2003; 76: pp. S118-S127.
22. Golman K., Zandt R.I., Lerche M., et. al.: Metabolic imaging by hyperpolarized 13C magnetic resonance imaging for in vivo tumor diagnosis. Cancer Res 2006; 66: pp. 10855-10860.
23. Schroeder M.A., Atherton H.J., Ball D.R., et. al.: Real-time assessment of Krebs cycle metabolism using hyperpolarized 13C magnetic resonance spectroscopy. FASEB J 2009; 23: pp. 2529-2538.
24. Schroeder M.A., Cochlin L.E., Heather L.C., et. al.: In vivo assessment of pyruvate dehydrogenase flux in the heart using hyperpolarized carbon-13 magnetic resonance. Proc Natl Acad Sci U S A 2008; 105: pp. 12051-12056.
25. Armstrong A., Brackenridge I., Jackson R.F.W., et. al.: A new method for the preparation of tertiary butyl ethers and esters. Tetrahedron Lett 1988; 29: pp. 2483-2486.
26. Ramsamy K., Olsen R.K., Emery T.: Synthesis of N-t-Boc-L-alpha -aminoadipic acid 1-t-butyl 6-ethyl ester from L-aspartic acid: a new route to L-alpha -aminoadipic acid. Synthesis 1982; pp. 42-43.
27. Ramalingam K., Woodard R.W.: Synthesis of stereospecific deuterium-labeled homoserines and homoserine lactones. J Org Chem 1988; 53: pp. 1900-1903.
28. Sutherland A., Willis C.L.: Synthesis of [6-13C]-L-lysine. J Lab Compds Radiopharm 1996; 38: pp. 95-102.
29. Hamilton D.J., Sutherland A.: A flexible approach for the synthesis of selectively labelled L-arginine. Tetrahedron Lett 2004; 45: pp. 5739-5741.
30. Moorthy J.N., Singhal N.: Facile and highly selective conversion of nitriles to amides via indirect acid-catalyzed hydration using TFA or AcOH-H2SO4. J Org Chem 2005; 70: pp. 1926-1929.
31. Wenner W.: Hydrolysis of arylacetonitriles. J Org Chem 1950; 15: pp. 548-551.
32. Studenov A.R., Szalda D.E., Ding Y.S.: Synthesis of no-carrier-added C-11 labeled D- and L-enantiomers of phenylalanine and tyrosine for comparative PET Studies. Nucl Med Biol 2003; 30: pp. 39-44.
33. Iranpoor N., Firouzabadi H., Akhlaghinia B., et. al.: Conversion of alcohols, thiols, and trimethylsilyl ethers to alkyl cyanides using triphenylphosphine/2,3-dichloro-5,6-dicyanobenzoquinone/n-Bu4NCN. J Org Chem 2004; 69: pp. 2562-2564.
34. Akhlaghinia B., Roohi E.: A new and convenient method of generating alkyl cyanides from alcohols and thiols using 2,4,6-trichloro[1,3,5]triazine/n-Bu4NCN. Lett Org Chem 2005; 2: pp. 725-730.
35. Board M., Humm S., Newsholme E.A.: Maximum activities of key enzymes of glycolysis, glutaminolysis, pentose phosphate pathway and tricarboxylic acid cycle in normal, neoplastic and suppressed cells. Biochem J 1990; 265: pp. 503-509.
36. Roberts E., Borges P.: Patterns of free amino acids in growing and regressing tumors. Cancer Res 1955; 15: pp. 697-699.
37. Barry-Billings M., Leighton B., Dimitriadis G.D., et. al.: The effect of tumour bearing on skeletal muscle glutamine metabolism. Int J Biochem 1991; 23: pp. 933-937.
38. Newsholme EA LA. Biochemistry for the Medical Sciences. Vol 1: John Wiley & Sons; 1984.
39. Adlington R.M., Baldwin J.E., Catterick D., et. al.: The synthesis of pyrimidin-4-yl substituted alpha -amino acids. A versatile approach from alkynyl ketones. J Chem Soc. Perkin Trans 1999; 1: pp. 855-866.
![Scheme 1, Synthesis of desired deuterated C-13 labeled [5- 13 C-4- 2 H 2 ]-L-glutamine, 8 .](https://storage.googleapis.com/dl.dentistrykey.com/clinical/FacileSynthesis513C42H2LGlutamineforHyperpolarizedMRSImagingofCancerCellMetabolism/0_1s20S1076633211002182.jpg)
![Figure 1, Time elapsed sequence of magnetic resonance spectroscopy signal after injection of hyperpolarized [5- 13 C-4- 2 H 2 ]-L-glutamine into the test tube containing SF188-Bcl-x L tumor cells. 13 C spectra were acquired with 20° pulses and a 4.5-second interpulse delay. The glutamine (Gln) signal was very high, and there was a rapid conversion of glutamine (Gln) to glutamate (Glu).](https://storage.googleapis.com/dl.dentistrykey.com/clinical/FacileSynthesis513C42H2LGlutamineforHyperpolarizedMRSImagingofCancerCellMetabolism/1_1s20S1076633211002182.jpg)