Hematopoietic malignancies that can be encountered in the breast include lymphoma, leukemia, and multiple myeloma/plasmacytoma. These are readily imaged via [18]F-fluorodeoxyglucose position emission tomography (PET)/computed tomography (CT) and can manifest as unilateral, bilateral, single, multiple, round/oval masses, or diffuse. These malignancies can occasionally mimic primary breast cancers. Conversely, benign conditions, such as the lactating breast can resemble hematopoietic malignancies of the breast. Although uncommon, familiarity with hematopoietic malignancies of the breast is important for proper interpretation of PET/CT. In this pictorial review, the PET/CT imaging features of patients with hematopoietic malignancies of the breast will be described, including pathology-proven cases of acute myelogenous leukemia, diffuse B-cell lymphoma, follicular lymphoma, acute myeloid leukemia with neutropenic granulocytic) sarcoma, and plasmacytoma. In addition, potential pitfalls will be discussed.
Hematopoietic neoplasms of the breast are rare lesions that are much less common than breast carcinoma. Hematopoietic malignancies of the breast include non-Hodgkin’s extranodal lymphoma, extramedullary leukemia, mainly acute myeloid leukemia with associated myeloid (granulocytic) sarcoma, and multiple myeloma/plasmacytoma, among other even less common neoplasms. These lesions may be primary, occurring only in the breast, versus more commonly secondary lesions. [18]F-fluorodeoxyglucose (FDG-18) position emission tomography (PET)/computed tomography (CT) has an important role in imaging hematopoietic malignancies . Overall, the sensitivity and specificity for initial staging of lymphoma is 87% and 100%, respectively . Similarly, PET/CT is a suitable modality evaluating the extent of multiple myeloma involvement, with a reported sensitivity of 85% and specificity of 92% . Furthermore, PET/CT is useful for differentiating post-treatment changes from residual tumor or recurrence . Although PET/CT is not indicated for assessment of leukemia, it can be helpful for cases of granulocytic sarcoma . In addition, PET/CT can effectively assess the extent of disease and help differentiate primary from secondary hematological breast malignancies. This modality is particularly helpful in patients with dense breast tissue. PET/CT is also an appropriate modality for evaluating breast lymphoma/leukemia treatment response . In addition, PET/CT can provide reliable prognostic information that is superior to other nuclear medicine studies and CT alone . The imaging features of a variety of hematopoietic malignancies of the breast are described and illustrated. In addition, potential pitfalls of imaging these neoplasms and their mimics on PET/CT are reviewed.
Lymphoma
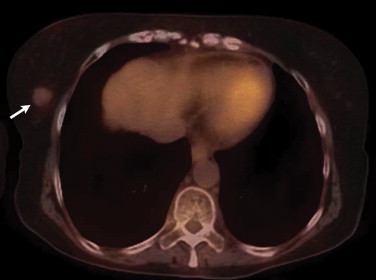
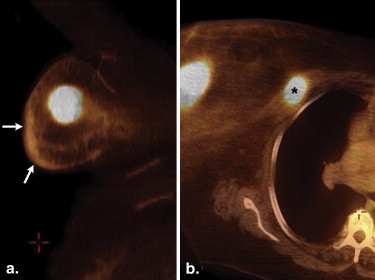
Overall, extranodal lymphomas constitute up to 40% of all lymphomas . However, involvement of the breast is unusual. Indeed, primary breast lymphomas comprise 0.04%–1.1% of all breast tumors and of 1.7%–2.2% of all extranodal non-Hodgkin’s lymphomas . The peak incidence of breast lymphoma is in the sixth and seventh decades . Most are intermediate or high grade . Large B-cell lymphoma is the most common subtype of non-Hodgkin’s to occur in the breast, comprising 40%–70% of breast lymphomas . Most primary breast lymphomas present as solitary masses and, less commonly, as diffuse breast enlargement . These tumors can be associated with skin changes, such as hypermetabolic skin thickening that can resemble inflammatory breast carcinomas ( Fig 1 ). The presence of axillary lymph node enlargement, hilar adenopathy, or adenopathy elsewhere may be a prominent feature of secondary breast lymphomas.
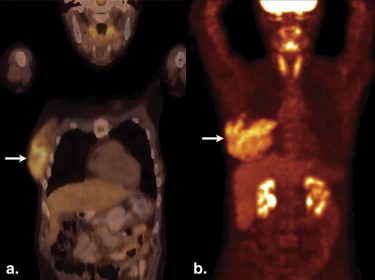
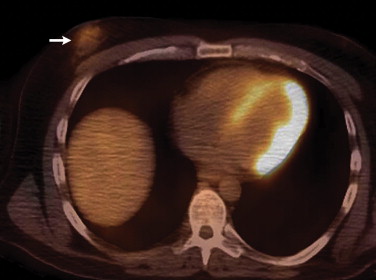
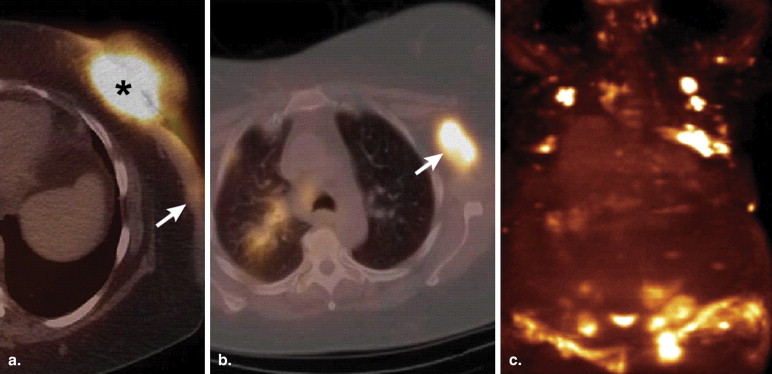
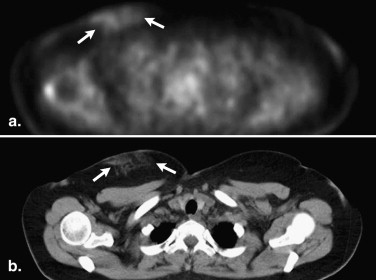
Follicular lymphoma is the second most common histological subtype of extranodal breast lymphoma . On PET, these neoplasms may manifest as ill-defined foci of hypermetabolism with infiltrative changes on the corresponding CT ( Fig 2 ). In general, non-Hodgkin’s lymphomas measure less than 3 cm in diameter and lack calcifications, which can help discern these from breast carcinomas, although no imaging features are pathognomonic. Uptake is typically homogeneous and strong, with a reported average standard uptake value of 10.6 . Alternatively, a “ring-shaped” pattern of hypermetabolism is sometimes encountered, particularly in rapidly growing or large tumors . Central necrosis or hemorrhage is likely responsible for this appearance. In contrast to primary breast lymphomas, up to 36% of secondary neoplasms are multiple and well-defined . Burkitt’s lymphoma of the breast is a distinctly rare condition, but may be related to pregnancy . This entity characteristically involves the bilateral breasts diffusely and tends to occur in younger women . Similar to other types of breast lymphomas, avid FDG uptake is apparent on PET ( Fig 3 ). These tumors may also be inconspicuous on ultrasound and mammography . Hodgkin’s lymphoma in the breast is even more unusual and its features on PET/CT are not well-characterized. Mucosa-associated lymphoid tissue lymphoma is a low-grade malignancy that has a reported incidence ranging from 0% to 64.3% among various series . These lesions may be bilateral as well .
Leukemia
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Multiple myeloma/plasmacytoma
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Potential pitfalls and mimics
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Conclusion
Get Radiology Tree app to read full this article<
References
1. Imataki O., Tamai Y., Yokoe K., et. al.: The utility of FDG-PET for managing patients with malignant lymphoma: analysis of data from a single cancer center. Intern Med 2009; 48: pp. 1509-1513.
2. Podoloff D.A., Macapinlac H.A.: PET and PET/CT in management of the lymphomas. Radiol Clin North Am 2007; 45: pp. 689-696. vii
3. Aschoff P., Häntschel M., Oksüz M., et. al.: Integrated FDG-PET/CT for detection, therapy monitoring and follow-up of granulocytic sarcoma. Nuklearmedizin 2009; 48: pp. 185-191.
4. Lütje S., de Rooy J.W., Croockewit S., et. al.: Role of radiography, MRI and FDGPET/CT in diagnosing, staging and therapeutical evaluation of patients with multiple myeloma. Ann Hematol 2009; 88: pp. 1161-1168.
5. Bredella M.A., Steinbach L., Caputo G., et. al.: Value of FDG PET in the assessment of patients with multiple myeloma. AJR Am J Roentgenol 2005; 184: pp. 1199-1204.
6. Kumar R., Xiu Y., Dhurairaj T., et. al.: F-18 FDG positron emission tomography in non- Hodgkin lymphoma of the breast. Clin Nucl Med 2005; 30: pp. 246-248.
7. Yang W.T., Lane D.L., Le-Petross H.T., et. al.: Breast lymphoma: imaging findings of 32 tumors in 27 patients. Radiology 2007; 245: pp. 692-702.
8. Becherer A., Jaeger U., Szabo M., et. al.: Prognostic value of FDG-PET in malignant lymphoma. Q J Nucl Med 2003; 47: pp. 14-21.
9. Chua S.C., Rozalli F.I., O’Connor S.R.: Imaging features of primary extranodal lymphomas. Clin Radiol 2009; 64: pp. 574-588.
10. Gholam D., Bibeau F., El Weshi A., et. al.: Primary breast lymphoma. Leuk Lymphoma 2003; 44: pp. 1173-1178.
11. Lin Y., Govindan R., Hess J.L.: Malignant hematopoietic breast tumors. Am J Clin Pathol 1997; 107: pp. 177-186.
12. Brogi E., Harris N.L.: Lymphomas of the breast: pathology and clinical behavior. Semin Oncol 1999; 26: pp. 357-364.
13. Zack J.R., Trevisan S.G., Gupta M.: Primary breast lymphoma originating in a benign intramammary lymph node. AJR Am J Roentgenol 2001; 177: pp. 177-178.
14. Bakheet S.M., Bakheet R., Ezzat A., et. al.: F-18 FDG positron emission tomography in primary breast non-Hodgkin’s lymphoma. Clin Nucl Med 2001; 26: pp. 299-301.
15. Sabate J.M., Clotet M., Torrubia S., et. al.: Radiologic evaluation of breast disorders related to pregnancy and lactation. Radiographics 2007; 27: pp. S101-S124.
16. Kyoung Jung H., Kim E.K., Yun M., et. al.: Bilateral breasts involvement in Burkitt’s lymphoma detected only by FDG-PET. Clin Imaging 2006; 30: pp. 57-59.
17. Farinha P., André S., Cabeçadas J., et. al.: High frequency of MALT lymphoma in a series of 14 cases of primary breast lymphoma. Appl Immunohistochem Mol Morphol 2002; 10: pp. 115-120.
18. Rajendran R.R., Palazzo J.P., Schwartz G.F., et. al.: Primary mucosa-associated lymphoid tissue lymphoma of the breast. Clin Breast Cancer 2008; 8: pp. 187-188.
19. Gopal S., Awasthi S., Elghetany M.T.: Bilateral breast MALT lymphoma: a case report and review of the literature. Ann Hematol 2000; 79: pp. 86-89.
20. Fitoz S., Atasoy C., Yavuz K., et. al.: Granulocytic sarcoma. Cranial and breast involvement. Clin Imaging 2002; 26: pp. 166-169.
21. Valbuena J.R., Admirand J.H., Gualco G., et. al.: Myeloid sarcoma involving the breast. Arch Pathol Lab Med 2005; 129: pp. 32-38.
22. Shea B., Reddy V., Abbitt P., et. al.: Granulocytic sarcoma (chloroma) of the breast: a diagnostic dilemma and review of the literature. Breast J 2004; 10: pp. 48-53.
23. von Falck C., Laenger F., Knapp W.H., et. al.: F-18 FDG PET/CT showing bilateral breast involvement in acute myeloid leukemia relapse. Clin Nucl Med 2009; 34: pp. 713-715.
24. Ngu I.W., Sinclair E.C., Greenaway S., et. al.: Unusual presentation of granulocytic sarcoma in the breast: a case report and review of the literature. Diagn Cytopathol 2001; 24: pp. 53-57.
25. Kumar P.V., Vasei M., Daneshbod Y., et. al.: Breast myeloma: a report of 3 cases with fine needle aspiration cytologic findings. Acta Cytol 2005; 49: pp. 445-448.
26. Kaviani A., Djamali-Zavareie M., Noparast M., et. al.: Recurrence of primary extramedullary plasmacytoma in breast both simulating primary breast carcinoma. World J Surg Oncol 2004; 2: pp. 29.
27. Gupta A., Kumar L., Aaron M.: A case of plasmacytoma of the breast mimicking an inflammatory carcinoma. Clin Lymph Myeloma 2008; 8: pp. 191-192.
28. Oba M., Sasaki M., Ii T., et. al.: A case of lymphocytic mastopathy requiring differential diagnosis from primary breast lymphoma. Breast Cancer 2009; 16: pp. 141-146.
29. Levine P.H., Zamuco R., Yee H.: Role of fine-needle aspiration cytology in breast lymphoma. Diagn Cytopathol 2004; 30: pp. 332-340.
30. Shah V.I., Raju U., Chitale D., et. al.: False-negative core needle biopsies of the breast: an analysis of clinical, radiologic, and pathologic findings in 27 consecutive cases of missed breast cancer. Cancer 2003; 97: pp. 1824-1831.
31. Bolívar A.V., Alonso-Bartolomé P., García E.O., et. al.: Ultrasound-guided core needle biopsy of non-palpable breast lesions: a prospective analysis in 204 cases. Acta Radiol 2005; 46: pp. 690-695.
32. Zagouri F., Sergentanis T.N., Nonni A., et. al.: Secondary breast lymphoma diagnosed by vacuum-assisted breast biopsy: a case report. J Med Case Reports 2007; 1: pp. 113.
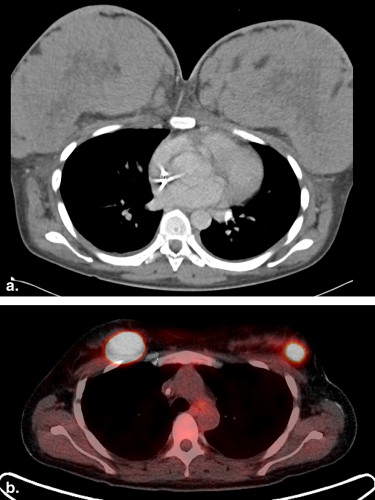
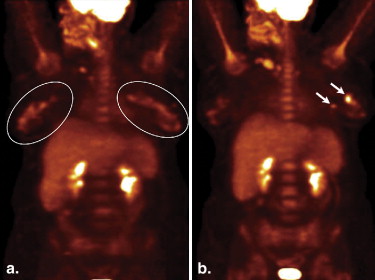
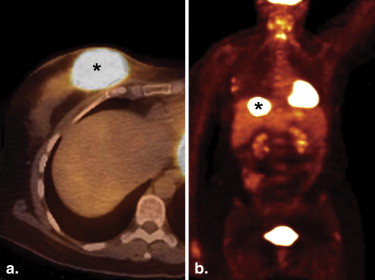
![Figure 5, A 62-year-old female with extramedullary acute myeloid leukemia manifesting as hypermetabolic foci involving the right parotid (standard uptake value [SUV] 9.7) ( open arrow ), an axillary lymph node (SUV 5.2), bilateral breasts (SUV up to 5.6) ( arrowheads ), soft tissue in the paravertebral region ( arrow ), and muscles ( encircled ) on the coronal positron emission tomography/computed tomography (a) fusion and MIP (b) images.](https://storage.googleapis.com/dl.dentistrykey.com/clinical/FDGPETCTManifestationsofHematopoieticMalignanciesoftheBreast/4_1s20S1076633210001832.jpg)