Rationale and Objectives
Multidetector-row computed tomography (MDCT) has evolved into a sensitive diagnostic tool for the noninvasive detection of coronary artery stenosis, but remains limited by spatial resolution. Flat panel volume computed tomography (fpVCT) offers a higher spatial resolution. In a postmortem investigation of autopsy specimens, the accuracies of fpVCT for measuring the severity of coronary artery stenosis and the size of atherosclerotic plaque components were determined.
Methods and Materials
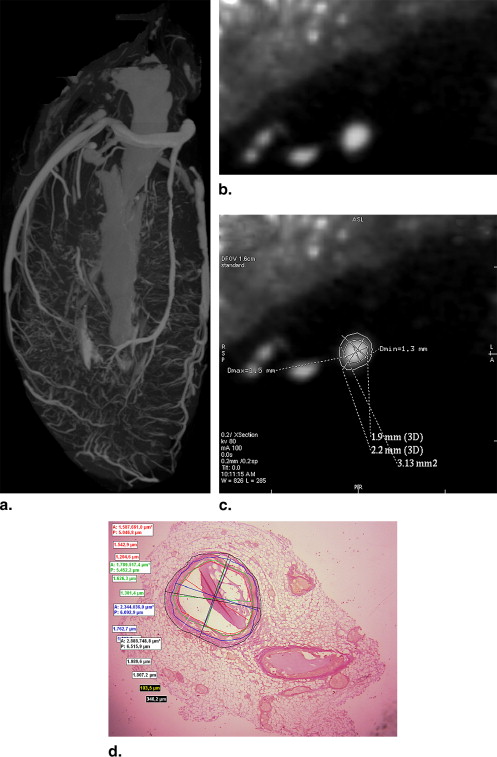
In 25 autopsy cases, hearts were isolated, the left anterior descending coronary arteries filled with contrast agent, and depicted with a prototype fpVCT unit with a slice thickness of 0.25 mm. Transections of the left anterior descending coronary arteries were reconstructed and compared with histopathologic sections using light microscopy.
Results
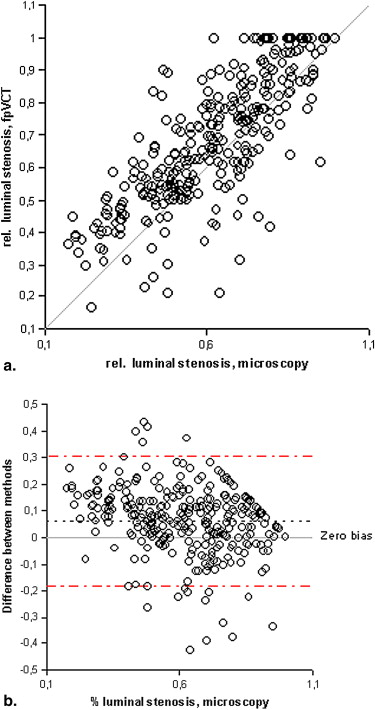
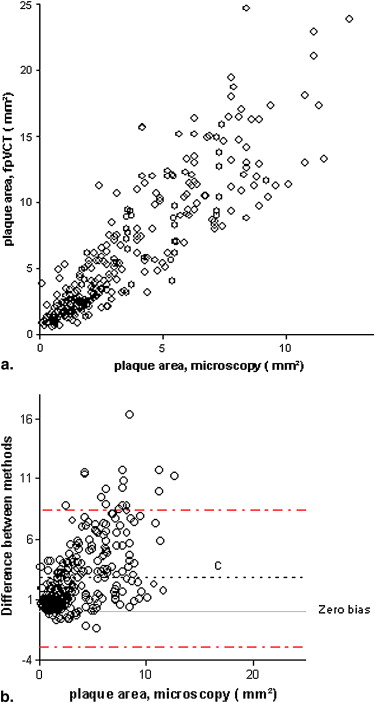
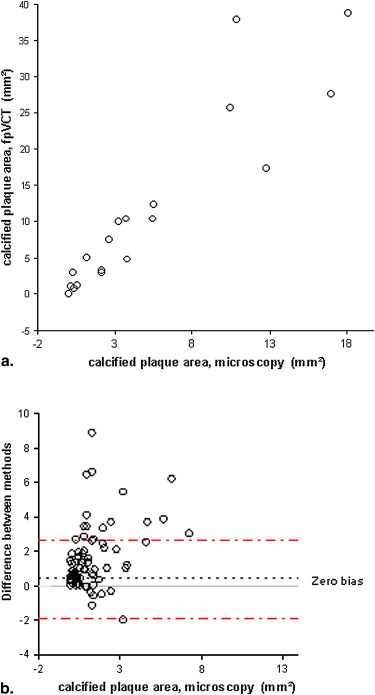
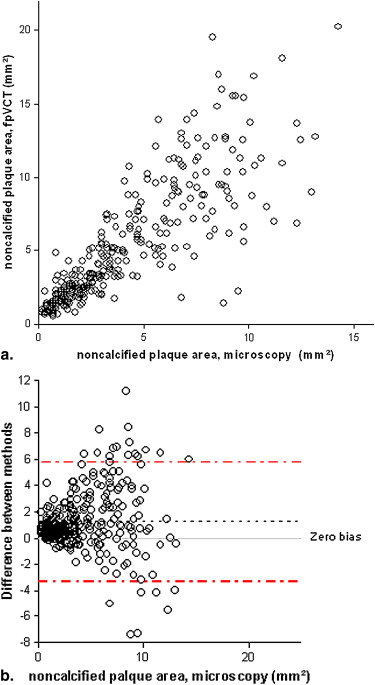
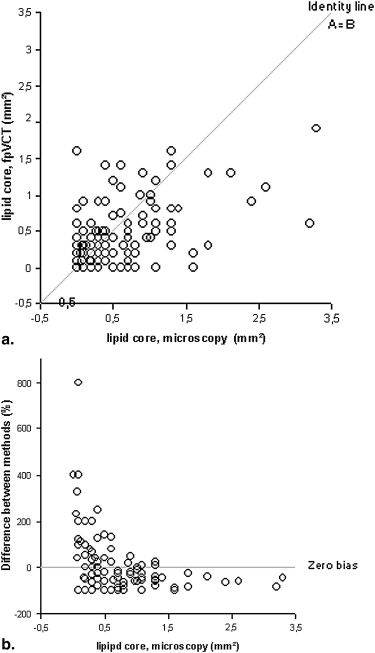
FpVCT measurements of luminal stenosis ( r = 0.81), total plaque area ( r = 0.88), calcified plaque area ( r = 0.92), noncalcified plaque area ( r = 0.83), and lipid core size ( r = 0.67; P < .02) correlated well with histopathology ( P < .0001). The limits of agreement for measuring any plaque component were three times smaller than those reported for MDCT.
Conclusions
Postmortem coronary fpVCT provides an accurate and reproducible method for the quantitative assessment of both luminal stenosis and atherosclerotic plaque size. Because of its high spatial resolution, the method should be sufficiently accurate to reliably detect the lipid pools of vulnerable plaques.
Multirow-detector computed tomography (MDCT) has evolved into a potential noninvasive tool for the detection of coronary artery stenosis and its use is regarded as appropriate in certain clinical scenarios . However, grading stenosis remains inaccurate in comparison to catheter angiography . The detection of severe stenosis is still insufficient, both on the vessel and the segment levels, even with the most recent 64-row MDCT technology . These important shortcomings have been ascribed to a lack of spatial resolution, since even the most advanced 64-row MDCT units have a slice thickness, as defined by detector element size in the z-axis direction, of no less than 0.5–0.75 mm, which is only a fraction of the spatial resolution of conventional coronary angiography of about 0.2 mm. A voxel resolution of 0.63 mm implies that a normal distal coronary artery lumen of 1.5 mm diameter is represented by no more than two to three computed tomography (CT) voxels, which limits the accuracy of grading stenoses. MDCT has also been assessed for its potential for identifying the vulnerable plaque in the future , and its spatial resolution has been found to limit its accuracy in this application as well .
Use of high-resolution flat panel volume computed tomography (fpVCT) detectors has been suggested as one method to improve the spatial resolution, and prototype CT units based on flat panel technology have become available .
Get Radiology Tree app to read full this article<
Methods
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Results
Get Radiology Tree app to read full this article<
Assessment of Coronary Stenosis
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Table 1
Reproducibility of Measuring Coronary Stenosis Severity
Parameter Method Correlation Coefficient r = Median Difference (mm 2 ) 95% Limits of Agreement (mm 2 ) Total vessel fpVCT 0.86 5.7 −6; 18 Histo 0.98 0.35 −1.6; 2.3 Lumen area fpVCT 0.76 −0.31 −3.8; 3.2 Histo 0.99 −0.02 −0.6; 0.6 % stenosis fpVCT 0.9 0.01 −0.17; 0.15 Histo 0.93 0.01 −0.11; 0.14
Histo: histology; fpVCT: flat panel volume computed tomography.
Get Radiology Tree app to read full this article<
Assessment of Plaque Composition
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Table 2
Reproducibility of Plaque Area Measurements from Two Independent Observers
Plaque Area Method Correlation Coefficient r = Median Difference (mm 2 ) 95% Limits of Agreement (mm 2 ) Total fpVCT 0.87 −0.2 −5.5; 5.1 Histo 0.9 −1.6 −5.4; 2.3 Calcified fpVCT 0.75 −0.004 −2.6; 2.6 Histo 0.79 0.07 −1.1; 1.2 Noncalcified fpVCT 0.8 −0.03 −5.2; 5.2 Histo 0.93 −0.29 −2.9; 2.3 Lipid core fpVCT 0.39 −0.19 −1.5; 1.1 Histo 0.45 −0.12 −1.5; 1.1
Histo: histology; fpVCT: flat panel volume computed tomography.
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Table 3
Comparison of fpVCT Plaque Measurements with Histology on a Per-vessel Basis
Plaque Area Method Median (25; 75% CI) (mm 2 ) Correlation Coefficient r = Median Difference (mm 2 ) 95% CI (mm 2 ) Total fpVCT 98 (68; 122) Histo 55 (43; 70) 0.95 41 −6; 88 Calcified fpVCT 6.2 (2.6; 16) Histo 2.9 (0.5; 9.3) 0.92 6 −8; 20 Noncalcified fpVCT 83 (65; 105) Histo 63 (50; 83) 0.94 18 −10; 41 Lipid core fpVCT 2.6 (0.6; 4.5) Histo 3.2 (0.8; 5.8) 0.69 −0.8 −5.2; 3.5
Histo: histology; fpVCT: flat panel volume computed tomography.
Table 4
Reproducibility of Plaque Area Measurements from Two Independent Observers on a Per-vessel Basis
Plaque Area Method Correlation Coefficient r = Median Difference (mm 2 ) 95% Limits of Agreement (mm 2 ) Total fpVCT 0.94 3.2 −28; 35 Histo 0.93 23 −9; 55 Calcified fpVCT 0.96 0.05 −7.2; 7.4 Histo 0.74 −1 −8.6; 6.6 Noncalcified fpVCT 0.93 0.4 −27; 28 Histo 0.94 4.2 −17; 26 Lipid core fpVCT 0.67 2.7 −2.6; 8.1 Histo 0.51 1.8 −5.7; 9.3
Histo: histology; fpVCT: flat panel volume computed tomography.
Get Radiology Tree app to read full this article<
Image Noise and Contrast
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Discussion
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Quantitative fpVCT Measurements of Coronary Artery Stenosis Severity
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Quantitative fpVCT Measurements of Atherosclerotic Plaque Components
Comparison with coronary angiography
Get Radiology Tree app to read full this article<
Comparison with 16- and 64-row MDCT
Get Radiology Tree app to read full this article<
Comparison with intracoronary ultrasound
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Clinical Implications
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
References
1. Dewey M., Rutsch W., Schnapauff D., et. al.: Coronary artery stenosis quantification using multislice computed tomography. Invest Radiol 2007; 42: pp. 78-84.
2. Herzog C., Zwerner P.L., Doll J.R., et. al.: Significant coronary artery stenosis: comparison of per-patient and per-vessel or per-segment basis at 64-section CT angiography. Radiology 2007; 244: pp. 112-120.
3. Hendel R.C., Kramer C.M., Patel M.R., et. al.: ACCF/ACR/SCCT/SCMR/ASNC/NASCI/SCAI/SIR 2006 Appropriateness criteria for cardiac computed tomography and cardiac magnetic resonance imaging. J Am Coll Cardiol 2006; 48: pp. 1475-1497.
4. Budoff M.J., Achenbach S., Blumenthal R.S., et. al.: Assessment of coronary artery disease by cardiac computed tomography: a scientific statement from the American Heart Association committee on cardiovascular imaging and intervention, council on cardiovascular radiology and intervention, and committee on cardiac imaging, council on clinical cardiology. Circulation 2006; 114: pp. 1761-1791.
5. Moselewski F., Ropers D., Pohle K., et. al.: Comparison of measurement of cross-sectional coronary atherosclerotic plaque and vessel areas by 16-slice multidetector computed tomography versus intravascular ultrasound. Am J Cardiol 2004; 94: pp. 1294-1297.
6. Carracosa P.M., Capunay C.M., Garcia-Merletti P., et. al.: Characterization of coronary atherosclerotic plaques by multidetector computed tomography. Am J Cardiol 2006; 97: pp. 598-602.
7. Nikolaou K., Becker C.R., Muders M., et. al.: Multidetector-row computed tomography and magnetic resonance imaging of atherosclerotic lesions in human ex vivo coronary arteries. Atherosclerosis 2004; 174: pp. 243-252.
8. Schroeder S., Kuettner A., Leitritz M., et. al.: Reliability of differentiating human coronary plaque morphology using contrast-enhanced multislice spiral computed tomography. J Comput Assist Tomogr 2004; 28: pp. 449-454.
9. Achenbach S., Moselewski F., Ropers D., et. al.: Detection of calcified and noncalcified coronary atherosclerotic plaque by contrast-enhanced, submillimeter multidetector spiral computed tomography. Circulation 2004; 109: pp. 14-17.
10. Knollmann F., Ducke F., Krist L., et. al.: Quantification of atherosclerotic plaque components by submillimeter computed tomography. Int J Cardiovasc Imag 2008; 24: pp. 301-310.
11. Gupta R., Grasruck M., Suess C., et. al.: Ultra-high resolution flat panel volume CT: fundamental principles, design architecture, and system characterization. Eur Radiol 2006; 16: pp. 1191-1205.
12. Knollmann F., Pfoh A.: Images in cardiovascular medicine: coronary artery imaging with flat-panel computed tomography. Circulation 2003; 107: pp. 1209.
13. Cademartiri F., Mollet N.R., Runza G., et. al.: Influence of intracoronary attenuation on coronary plaque measurements using multislice computed tomography: observations in an ex vivo model of coronary computed tomography angiography. Eur Radiol 2005; 15: pp. 1426-1431.
14. Halliburton S.S., Schoenhagen P., Nair A., et. al.: Contrast enhancement of coronary atherosclerotic plaque: a high-resolution, multidetector-row computed tomography study of pressure-perfused, human ex-vivo coronary arteries. Coron Artery Dis 2006; 17: pp. 553-560.
15. Horiguchi J., Fujioka C., Kiguchi M., et. al.: Soft and intermediate plaques in coronary arteries: how accurately can we measure CT attenuation using 64-MDCT?. AJR Am J Roentgenol 2007; 189: pp. 981-988.
16. Bland J.M., Altman D.G.: Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986; pp. 307-310.
17. Krouwer J.S.: Why Bland-Altman plots should use X, not (Y + X)/2 when X is a reference method. Stat Med 2008; 27: pp. 778-780.
18. Kefer J., Coche E., Legros G., et. al.: Head-to-head comparison of three-dimensional navigator-gated magnetic resonance imaging and 16-slice computed tomography to detect coronary artery stenosis in patients. J Am Coll Cardiol 2005; 46: pp. 92-100.
19. Cai J., Hatsukami T.S., Ferguson M.S., et. al.: In vivo quantitative measurement of intact fibrous cap and lipid-rich necrotic core size in atherosclerotic carotid plaque: comparison of high-resolution, contrast-enhanced magnetic resonance imaging and histology. Circulation 2005; 112: pp. 3437-3444.
20. Worthley S.G., Helft G., Fuster V., et. al.: High resolution ex vivo magnetic resonance imaging of in situ coronary and aortic atherosclerotic plaque in a porcine model. Atherosclerosis 2000; 150: pp. 321-329.
21. Grondin C.M., Dyrda I., Pasternac A., et. al.: Discrepancies between cineangiographic and postmortem findings in patients with coronary artery disease and recent myocardial infarction. Circulation 1974; 49: pp. 703-708.
22. Eusterman J.H., Achor R.W.P., Kincaid O.W., et. al.: Atherosclerotic disease of the coronary arteries: a pathologic-radiologic correlative study. Circulation 1962; 26: pp. 1288-1295.
23. Zir L.M., Miller S.W., Dinsmore R.E., et. al.: Interobserver variability in coronary angiography. Circulation 1976; 53: pp. 627-632.
24. Popma J.J., Lansky A.J., Yeh W., et. al.: Reliability of the quantitative angiographic measurements in the new approaches to coronary intervention registry. Am J Cardiol 1997; 80: pp. 19K-25K.
25. Leber A.W., Knez A., von Ziegler F., et. al.: Quantification of obstructive and nonobstructive coronary lesions by 64-slice computed tomography. J Am Coll Cardiol 2005; 46: pp. 147-154.
26. Schroeder S., Kopp A., Ohnesorge B., et. al.: Accuracy and reliability of quantitative measurements in coronary arteries by multi-slice computed tomography. Clin Radiol 2001; 56: pp. 466-476.
27. Cury R.C., Ferencik M., Achenbach S., et. al.: Accuracy of 16-slice multi-detector CT to quantify the degree of coronary artery stenosis: assessment of cross-sectional and longitudinal vessel reconstructions. Eur J Radiol 2006; 57: pp. 345-350.
28. Leber A.W., Johnson T., Becker A., et. al.: Diagnostic accuracy of dual-source multi-slice CT-coronary angiography in patients with an intermediate pretest likelihood for coronary artery disease. Eur Heart J 2007; 28: pp. 2354-2360.
29. Raff G.L., Gallagher M.J., O’Neill W.W., et. al.: Diagnostic accuracy of noninvasive coronary angiography using 64-slice spiral computed tomography. J Am Coll Cardiol 2005; 46: pp. 552-557.
30. Mahnken A.H., Seyfahrt T., Flohr T., et. al.: Flat-panel detector computed tomography for the assessment of coronary artery stents. Invest Radiol 2005; 40: pp. 8-13.
31. Potkin B.N., Bartorelli A.L., Gessert J.M., et. al.: Coronary artery imaging with intravascular high-frequency ultrasound. Circulation 1990; 81: pp. 1575-1585.
32. Tobis J.M., Mallery J., Mahon D., et. al.: Intravascular ultrasound imaging of human coronary arteries in vivo. Circulation 1991; 83: pp. 913-926.
33. Sarwar A., Rieber J., Mooyaart E.A.Q., et. al.: Calcified plaque: measurement of area at thin section flat panel CT and 64-section multidetector CT and comparison with histopathologic findings. Radiology 2008; 249: pp. 301-306.
34. Virmani R., Burke A.P., Farb A., et. al.: Pathology of the vulnerable plaque. J Am Coll Cardiol 2006; 47: pp. C13-C18.
35. Mintz G.S., Nissen S.E., Anderson W., et. al.: American college of cardiology clinical expert consensus document on standards for acquisition, measurement and reporting of intravascular ultrasound studies (IVUS). J Am Coll Cardiol 2001; 37: pp. 1478-1492.
36. Smith S.C., Dove Jacobs A.K., Kennedy J.W., et. al.: ACC/AHA guidelines for percutaneous coronary intervention. J Am Coll Cardiol 2001; 37: pp. 2399.
37. Pijls N.H.J., De Bruyne B., Peels K., et. al.: Measurement of fractional flow reserve to assess the functional severity of coronary-artery stenoses. N Engl J Med 1996; 334: pp. 1703-1708.
38. Gould K.L.: Quantification of coronary artery stenosis in vivo. Circ Res 1985; 57: pp. 341-353.
39. Kern M.J., Lerman A., Bech J.-W., et. al.: Physiological assessment of coronary artery disease in the cardiac catheterization laboratory. Circulation 2006; 114: pp. 1321-1341.
40. Boden W.E., O’Rourke R.A., Teo K., et. al.: Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med 2007; 356: pp. 1503-1516.
41. Greschus S., Kiessling F., Lichy M.P., et. al.: Potential applications of flat-panel volumetric CT in morphologic and functional small animal imaging. Neoplasia 2005; 7: pp. 730-740.