Rationale and Objectives
Imaging tumor response to neoadjuvant chemotherapy in vivo offers unique opportunities for patient care and clinical decision-making. Detailed imaging studies may allow oncologists to optimize therapeutic drug type and dose based on individual patient response. Most radiologic methods are used sparingly because of cost; thus, important functional information about tumor response dynamics may be missed. In addition, current clinical standards are based on determining tumor size changes; thus, standard anatomic imaging may be insensitive to early or frequent biochemical responses. Because optical methods provide functional imaging end points, our objective is to develop a low-barrier-to-access bedside approach that can be used for frequent, functional assessment of dynamic tumor physiology in individual patients.
Materials and Methods
Diffuse Optical Spectroscopic Imaging (DOSI) is a noninvasive, bedside functional imaging technique that quantifies the concentration and molecular state of tissue hemoglobin, water, and lipid. Pilot clinical studies have shown that DOSI may be a useful tool for quantifying neoadjuvant chemotherapy response, typically by comparing the degree of change in tumor water and deoxy-hemoglobin concentration before and after therapy. Patient responses at 1 week and mid-therapy have been used to predict clinical outcome. In this report, we assess the potential value of frequent DOSI monitoring by performing measurements on 19 different days in a 51-year-old subject with infiltrating ductal carcinoma (initial tumor size 60 × 27 mm) who received neoadjuvant chemotherapy (anthracyclines and bevacizumab) over an 18-week period.
Results
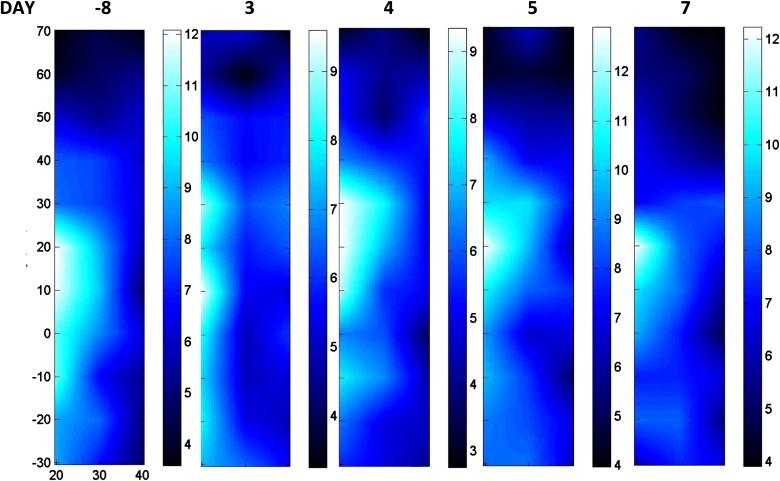
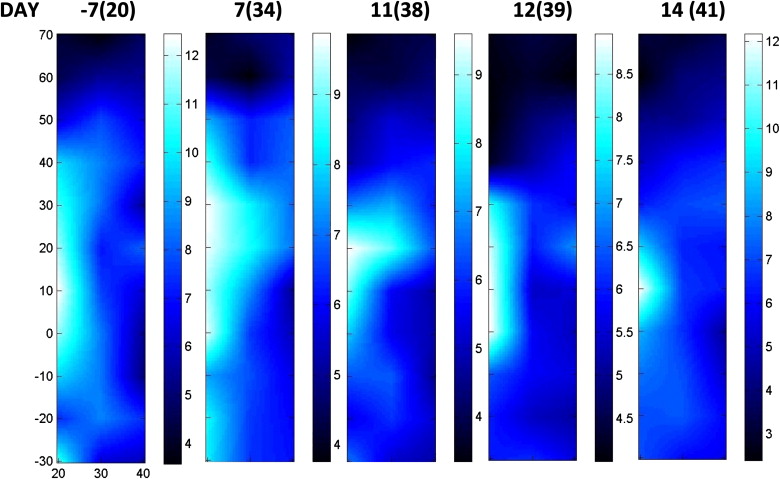
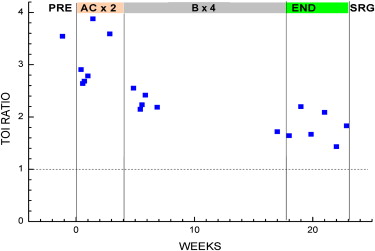
A composite index, the Tissue Optical Index (TOI), showed a significant (∼50%) decrease over the nearly 18 weeks of chemotherapy. Tumor response was sensitive to the type of chemotherapy agent, and functional indices fluctuated in a manner consistent with dynamic tumor physiology. Final pathology revealed 4 mm of residual disease, which was detectible by DOSI at the conclusion of chemotherapy before surgery.
Conclusion
This case study suggests that DOSI may be a bedside-capable tool for frequent longitudinal monitoring of therapeutic functional response to neoadjuvant chemotherapy.
Presurgical neoadjuvant chemotherapy (NAC) has an increasingly important role in breast cancer patient care and drug development . Also referred to as “preoperative systemic therapy,” NAC has become standard of care for locally advanced and inflammatory breast cancer . In this setting, chemotherapy is administered before surgery to improve breast tissue conservation by reducing tumor size . Additional important benefits compared to adjuvant chemotherapy include the ability to directly assess an individual’s sensitivity to specific drugs, and initiating treatment of possible nodes and metastases before surgery.
Imaging individual tumor response can provide sufficient information for oncologists to alter therapeutics with a goal of improving overall survival, decreasing cancer recurrence, and minimizing exposure to ineffective drugs. Several groups are developing advanced imaging techniques for monitoring the effects of neoadjuvant chemotherapy, using for example magnetic resonance imaging (MRI) and spectroscopy (MRS), and positron emission tomography (PET) . These studies provide compelling evidence that functional imaging can be used to monitor and perhaps even predict therapeutic outcome . However, it is unclear what are the most sensitive and informative imaging end points and how often they should be assessed.
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Materials and methods
Instrumentation
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
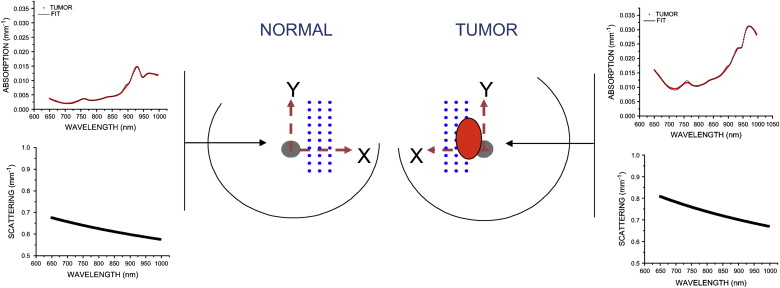
Measured Information Content
Get Radiology Tree app to read full this article<
DOSI Measurement Technique
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Image Analysis
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Patient Selection
Get Radiology Tree app to read full this article<
Measurement and Treatment Schedules
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Results
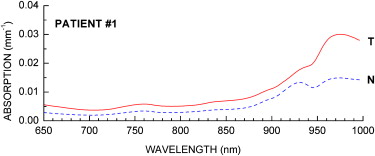
Baseline Tumor NIR Absorption Spectra
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Table 1
Tumor Functional Properties Measured by DOSI before Chemotherapy
ctO 2 Hb (μM) ctHHb (μM) ctH 2 O (%) Lipid (%) TOI Tumor 15.0 ± 1.0 9.0 ± 1.2 40.7 ± 5.9 51.4 ± 4.2 7.7 ± 2.8 Normal 13.0 ± 2.2 6.0 ± 1.1 23.0 ± 6.3 71.5 ± 9.5 2.2 ± 1.1
DOSI, Diffuse Optical Spectroscopic Imaging; ctO 2 Hb, tissue concentration of oxy-hemoglobin; ctHHb, tissue concentration of deoxy-hemoglobin; ctH 2 O, tissue concentration of water; TOI, tissue optical index.
Get Radiology Tree app to read full this article<
Initial A/C Chemotherapy Stage
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Bevacizumab Therapy Stage
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Entire Therapy Sequence
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Discussion
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Conclusion
Get Radiology Tree app to read full this article<
Acknowledgments
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
References
1. Wolff A.C., Berry D., Carey L.A., et. al.: Research issues affecting preoperative systemic therapy for operable breast cancer. J Clin Oncol 2008; 26: pp. 806-813.
2. Gralow J.R., Zujewski J.A., Winer E.: Preoperative therapy in invasive breast cancer: reviewing the state of the science and exploring new research directions. J Clin Oncol 2008; 26: pp. 696-697.
3. Jones R.L., Smith I.E.: Neoadjuvant treatment for early-stage breast cancer: opportunities to assess tumour response. Lancet Oncol 2006; 7: pp. 869-874.
4. Fisher B., Brown A., Mamounas E., et. al.: Effect of preoperative chemotherapy on local-regional disease in women with operable breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-18. J Clin Oncol 1997; 15: pp. 2483-2493.
5. Bahri S., Chen J.H., Mehta R.S., et. al.: Residual breast cancer diagnosed by MRI in patients receiving neoadjuvant chemotherapy with and without bevacizumab. Ann Surg Oncol 2009; 16: pp. 1619-1628.
6. Baek H.M., Chen J.H., Nie K., et. al.: Predicting pathologic response to neoadjuvant chemotherapy in breast cancer by using MR imaging and quantitative 1H MR spectroscopy. Radiology 2009; 251: pp. 653-662.
7. Zakhireh J., Gomez R., Esserman L.: Converting evidence to practice: a guide for the clinical application of MRI for the screening and management of breast cancer. Eur J Cancer 2008; 44: pp. 2742-2752.
8. Ah-See M.L., Makris A., Taylor N.J., et. al.: Early changes in functional dynamic magnetic resonance imaging predict for pathologic response to neoadjuvant chemotherapy in primary breast cancer. Clin Cancer Res 2008; 14: pp. 6580-6589.
9. Meisamy S., Bolan P.J., Baker E.H., et. al.: Neoadjuvant chemotherapy of locally advanced breast cancer: predicting response with in vivo (1)H MR spectroscopy—a pilot study at 4 T. Radiology 2004; 233: pp. 424-431.
10. Martincich L., Montemurro F., De Rosa G., et. al.: Monitoring response to primary chemotherapy in breast cancer using dynamic contrast-enhanced magnetic resonance imaging. Breast Cancer Res Treat 2004; 83: pp. 67-76.
11. Partridge S.C., Gibbs J.E., Lu Y., et. al.: MRI measurements of breast tumor volume predict response to neoadjuvant chemotherapy and recurrence-free survival. AJR Am J Roentgenol 2005; 184: pp. 1774-1781.
12. Duch J., Fuster D., Munoz M., et. al.: (18)F-FDG PET/CT for early prediction of response to neoadjuvant chemotherapy in breast cancer. Eur J Nucl Med Mol Imaging 2009; 36: pp. 1551-1557.
13. Emmering J., Krak N.C., Van der Hoeven J.J., et. al.: Preoperative [18F] FDG-PET after chemotherapy in locally advanced breast cancer: prognostic value as compared with histopathology. Ann Oncol 2008; 19: pp. 1573-1577.
14. Mankoff D.A., Dunnwald L.K., Gralow J.R., et. al.: Changes in blood flow and metabolism in locally advanced breast cancer treated with neoadjuvant chemotherapy. J Nucl Med 2003; 44: pp. 1806-1814.
15. Schelling M., Avril N., Nahrig J., et. al.: Positron emission tomography using [(18)F]Fluorodeoxyglucose for monitoring primary chemotherapy in breast cancer. J Clin Oncol 2000; 18: pp. 1689-1695.
16. Mankoff D.A., O’Sullivan F., Barlow W.E., et. al.: Molecular imaging research in the outcomes era: measuring outcomes for individualized cancer therapy. Acad Radiol 2007; 14: pp. 398-405.
17. Tromberg B.J., Pogue B.W., Paulsen K.D., et. al.: Assessing the future of diffuse optical imaging technologies for breast cancer management. Med Phys 2008; 35: pp. 2443-2451.
18. Cerussi A., Shah N., Hsiang D., et. al.: In vivo absorption, scattering, and physiologic properties of 58 malignant breast tumors determined by broadband diffuse optical spectroscopy. J Biomed Opt 2006; pp. 11. 044005
19. Kukreti S., Cerussi A., Tromberg B., et. al.: Intrinsic tumor biomarkers revealed by novel double-differential spectroscopic analysis of near-infrared spectra. J Biomed Opt 2007; pp. 12. 020509
20. Chung S.H., Cerussi A.E., Klifa C., et. al.: In vivo water state measurements in breast cancer using broadband diffuse optical spectroscopy. Phys Med Biol 2008; 53: pp. 6713-6727.
21. Jakubowski D.B., Cerussi A.E., Bevilacqua F., et. al.: Monitoring neoadjuvant chemotherapy in breast cancer using quantitative diffuse optical spectroscopy: a case study. J Biomed Opt 2004; 9: pp. 230-238.
22. Zhu Q., Kurtzma S.H., Hegde P., et. al.: Utilizing optical tomography with ultrasound localization to image heterogeneous hemoglobin distribution in large breast cancers. Neoplasia 2005; 7: pp. 263-270.
23. Shah N., Gibbs J., Wolverton D., et. al.: Combined diffuse optical spectroscopy and contrast-enhanced magnetic resonance imaging for monitoring breast cancer neoadjuvant chemotherapy: a case study. J Biomed Opt 2005; 10: pp. 51503.
24. Choe R., Corlu A., Lee K., et. al.: Diffuse optical tomography of breast cancer during neoadjuvant chemotherapy: a case study with comparison to MRI. Med Phys 2005; 32: pp. 1128-1139.
25. Pham T.H., Coquoz O., Fishkin J.B., et. al.: Broad bandwidth frequency domain instrument for quantitative tissue optical spectroscopy. Rev Sci Instrum 2000; 71: pp. 2500-2513.
26. Bevilacqua F., Berger A.J., Cerussi A.E., et. al.: Broadband absorption spectroscopy in turbid media by combined frequency-domain and steady-state methods. Appl Opt 2000; 39: pp. 6498-6507.
27. Cerussi A.E., Jakubowski D., Shah N., et. al.: Spectroscopy enhances the information content of optical mammography. J Biomed Opt 2002; 7: pp. 60-71.
28. Mourant J.R., Fuselier T., Boyer J., et. al.: Predictions and measurements of scattering and absorption over broad wavelength ranges in tissue phantoms. Appl Opt 1997; 36: pp. 949-957.
29. Cerussi A., Siavoshi S., Durkin A., et. al.: Effect of contact force on breast tissue optical property measurements using a broadband diffuse optical spectroscopy handheld probe. Appl Opt 2009; 48: pp. 4270-4277.
30. Tromberg B.J., Shah N., Lanning R., et. al.: Non-invasive in vivo characterization of breast tumors using photon migration spectroscopy. Neoplasia 2000; 2: pp. 26-40.
31. Pogue B.W., Poplack S.P., McBride T.O., et. al.: Quantitative hemoglobin tomography with diffuse near-infrared spectroscopy: pilot results in the breast. Radiology 2001; 218: pp. 261-266.
32. Franceschini M.A., Moesta K.T., Fantini S., et. al.: Frequency-domain techniques enhance optical mammography: initial clinical results. Proc Natl Acad Sci U S A 1997; 94: pp. 6468-6473.
33. Chance B., Nioka S., Zhang J., et. al.: Breast cancer detection based on incremental biochemical and physiological properties of breast cancers: a six-year, two-site study. Acad Radiol 2005; 12: pp. 925-933.
34. Taroni P., Danesini G., Torricelli A., et. al.: Clinical trial of time-resolved scanning optical mammography at 4 wavelengths between 683 and 975 nm. J Biomed Opt 2004; 9: pp. 464-473.
35. Cerussi A., Hsiang D., Shah N., et. al.: Predicting response to breast cancer neoadjuvant chemotherapy using diffuse optical spectroscopy. Proc Natl Acad Sci U S A 2007; 104: pp. 4014-4019.
36. Casanovas O., Hicklin D.J., Bergers G., et. al.: Drug resistance by evasion of antiangiogenic targeting of VEGF signaling in late-stage pancreatic islet tumors. Cancer Cell 2005; 8: pp. 299-309.
37. Gandjbakhche A.H., Nossal R., Bonner R.F.: Resolution limits for optical transillumination of abnormalities deeply embedded in tissues. Med Phys 1994; 21: pp. 185-191.
38. Pogue B.W., Davis S.C., Song X., et. al.: Image analysis methods for diffuse optical tomography. J Biomed Opt 2006; 11: pp. 33001.
39. Li A., Liu J., Tanamai W., et. al.: Assessing the spatial extent of breast tumor intrinsic optical contrast using ultrasound and diffuse optical spectroscopy. J Biomed Opt 2008; pp. 13. 030504
40. Yeh E., Slanetz P., Kopans D.B., et. al.: Prospective comparison of mammography, sonography, and MRI in patients undergoing neoadjuvant chemotherapy for palpable breast cancer. AJR Am J Roentgenol 2005; 184: pp. 868-877.
41. Warren R.M., Bobrow L.G., Earl H.M., et. al.: Can breast MRI help in the management of women with breast cancer treated by neoadjuvant chemotherapy?. Br J Cancer 2004; 90: pp. 1349-1360.
42. No K.S., Kwong R., Chou P.H., et. al.: Design and testing of a miniature broadband frequency domain photon migration instrument. J Biomed Opt 2008; pp. 13. 050509
43. Boas D.A., O’Leary M.A., Chance B., et. al.: Detection and characterization of optical inhomogeneities with diffuse photon density waves: a signal-to-noise analysis. Appl Opt 1997; 36: pp. 75-92.