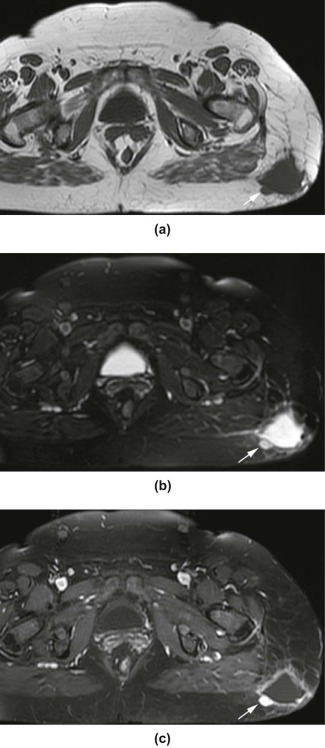
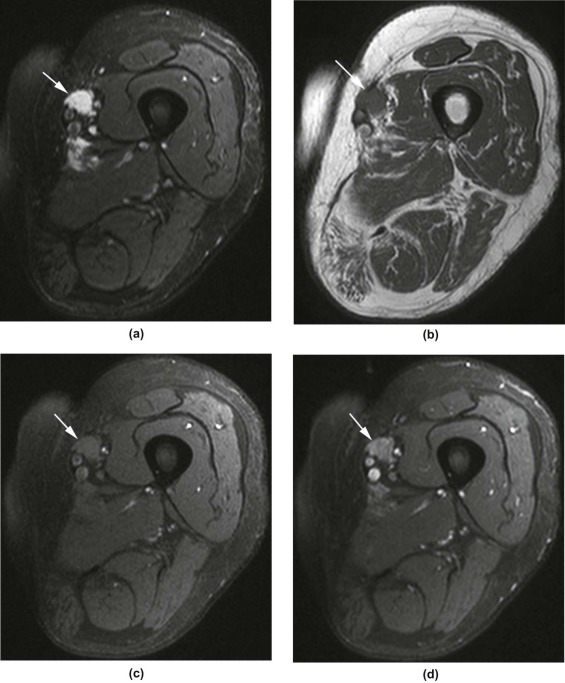
Rationale and Objectives
To determine how utilization of postgadolinium magnetic resonance imaging (MRI) influenced reader accuracy and confidence at identifying postoperative soft tissue sarcoma (STS) recurrence among readers with various levels of expertise.
Materials and Methods
This retrospective study was institutional review board approved and Health Insurance Portability and Accountability Act compliant. Postoperative MRI from 26 patients with prior STS resection (13 patients with confirmed recurrence, 13 without recurrence) was reviewed. Four blinded readers of varying expertise (radiology resident, fellow, attending, and orthopedic oncologist) initially evaluated only the precontrast images and rated each MRI for recurrence on a 5-point confidence scale. Assessment was repeated with the addition of contrast-enhanced sequences. Diagnostic accuracy based on confidence ratings was evaluated using the area under the receiver operating characteristic curve (AUC). Changes in confidence ratings were calculated using Wilcoxon signed-rank test.
Results
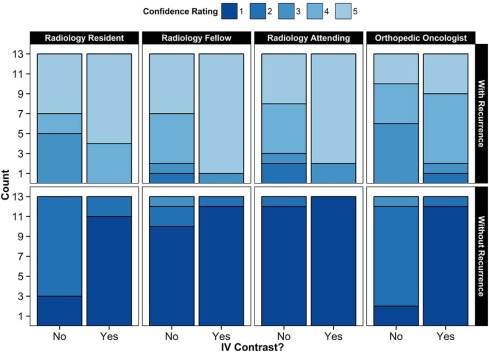
All readers demonstrated good diagnostic accuracy both with and without contrast-enhanced images (AUC >0.98 for each reader). When contrast-enhanced images were made available, the resident recorded improved confidence with both assigning ( P = 0.031) and excluding recurrence ( P = 0.006); the fellow showed improved confidence only with assigning recurrence ( P = 0.015); and the surgeon showed improved confidence in excluding recurrence ( P = 0.003). The addition of contrast-enhanced images did not significantly influence the diagnostic confidence of the attending radiologist.
Conclusions
Diagnostic accuracy of MRI was excellent in evaluating postoperative STS recurrence, and reader confidence improved depending on expertise when postgadolinium imaging was included in the assessment.
Introduction
Soft tissue sarcomas (STS) are a heterogeneous group of mesenchymal neoplasms that constitute less than 1% of all adult malignancies . Primary treatment of STS typically consists of limb-sparing surgical resection with or without radiation therapy and/or chemotherapy . The role of chemotherapy and radiation preoperatively and postoperatively can vary depending on specific diagnosis, adequacy of margin, tumor grade, and lesion location. Although the reported rates of local recurrence ranged from 6.5% to near 50% and averaging around 20% , there is no established optimal surveillance strategy given the lack of prospective data . In addition to periodic clinical assessments posttreatment, magnetic resonance imaging (MRI) is often performed to survey for local recurrence . It is the most appropriate radiologic procedure for local recurrence surveillance of malignant or aggressive musculoskeletal soft tissue tumors based on the American College of Radiology Appropriateness Criteria guideline . However, its utility in detecting asymptomatic recurrence has been disputed, with prior studies showing inconsistent results, and several recent studies suggesting doubtful benefits . Advanced imaging tools such as positron emission tomography hybrid imaging and functional MRI employing dynamic contrast enhancement (DCE) and diffusion-weighted imaging with apparent diffusion coefficient have shown promising results in supplementing standard MRI protocols, such as improved specificity by DCE, in identifying recurrence .
Intravenous gadolinium-based contrast agents are commonly used in MRI follow-up of STS after treatment . The American College of Radiology Appropriateness Criteria express only a mild preference for MRI with and without contrast over MRI without contrast in STS surveillance . To our knowledge, there is no study in the literature evaluating a reader’s ability to detect STS recurrence on postoperative MRI, which also explores the impact of contrast-enhanced pulse sequences, controlled for patients, readers, and techniques.
Get Radiology Tree app to read full this article<
Materials and Methods
Patient Selection and MRI Examinations
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Image Analysis
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Statistical Analysis
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Results
Get Radiology Tree app to read full this article<
TABLE 1
Patient Demographics and Resected Tumor Characteristics ( n = 26 Patients)
Variable No. (%) or Median (Range)P Value With Recurrence ( n = 13) Without Recurrence ( n = 13) Sex Male 8(62) 4(31) 0.24 Female 5(38) 9(69) Age, years 60(26–75) 53(27–85) 0.64 Tumor location Lower extremity 5(38) 9(69) 0.27 Pelvis 3(23) 3(23) Thorax 1(8) 0(0) Upper extremity 4(31) 1(8) Tumor depth Deep 10(77) 9(69) >0.99 In relation to superficial fascia Superficial 3(23) 4(31) Largest tumor dimension, cm Before surgery \* 7.2(3.0–17.0) 5.0(2.5–23.0) 0.51 Recurrence 5.0(1.0–23.0) — — Tumor margin † Positive 6(46) 4(31) 0.69 Negative 7(54) 8(62) Tumor grade ‡ Low 3(23) 2(15) 0.67 Intermediate 3(23) 5(38) High 7(54) 5(38) Tumor histopathology Myxoid type 8(62) 4(31) 0.24 Other 5(38) 9(69)
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
TABLE 2
Diagnostic Accuracy of Confidence Score for Recurrence with or without Postcontrast Images Available
Reader AUC Sensitivity † Specificity † With Contrast Without Contrast_P_ Value \* With Contrast Without Contrast_P_ Value \* With Contrast Without Contrast_P_ Value \* R1: Resident radiologist 1.000 1.000 >0.99 13(100.0) 8(61.5) 0.0625 13(100.0) 13(100.0) >0.99 R2: Fellow radiologist 1.000 0.985 0.060 12(92.3) 11(84.6) >0.99 13(100.0) 12(92.3) >0.99 R3: Attending radiologist 1.000 0.994 0.25 11(84.6) 10(76.9) >0.99 13(100.0) 13(100.0) >0.99 R4: Orthopedic oncologist 0.997 0.982 0.52 11(84.6) 7(53.9) 0.125 13(100.0) 12(92.3) >0.99 All readers(R1–R4) ‡ 0.999 0.990 0.19 47(90.4) 36(69.2) 0.027 52(100.0) 50(96.2) 0.50 Radiologists(R1–R3) ‡ 1.000 0.993 0.016 36(92.3) 29(74.4) 0.065 39(100.0) 38(97.4) >0.99
AUC, area under the ROC curve; ROC, receiver operating characteristic.
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
TABLE 3
Confidence in Recurrence Diagnosis
Reader With Recurrence Without Recurrence With Contrast Without Contrast_P_ Value \* With Contrast Without Contrast_P_ Value \* R1: Resident radiologist 4.7 ± 0.5 4.1 ± 1.0 0.031 1.2 ± 0.4 1.8 ± 0.4 0.006 R2: Fellow radiologist 4.8 ± 0.6 4.2 ± 0.9 0.015 1.1 ± 0.3 1.3 ± 0.6 0.15 R3: Attending radiologist 4.7 ± 0.8 4.0 ± 1.1 0.065 1.0 ± 0.0 1.1 ± 0.3 >0.99 R4: Orthopedic oncologist 4.1 ± 0.9 3.8 ± 0.8 0.13 1.1 ± 0.3 1.9 ± 0.5 0.003 R1–R4 combined 4.6 ± 0.7 4.0 ± 0.9 <0.001 1.1 ± 0.3 1.5 ± 0.6 <0.001 R1–R3 combined 4.7 ± 0.6 4.1 ± 1.0 <0.001 1.1 ± 0.3 1.4 ± 0.5 0.008
Values are mean ± standard deviation of the confidence scale (5-point scale with 1 = definitely absent and 5 = definitely present).
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Discussion
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
References
1. Clark M.A., Fisher C., Judson I., et. al.: Soft-tissue sarcomas in adults. N Engl J Med 2005; 353: pp. 701-711.
2. Siegel R., Naishadham D., Jemal A.: Cancer statistics, 2012. CA Cancer J Clin 2012; 62: pp. 10-29.
3. Fletcher C.D.Unni K.K.Mertens F.World Health Organization classification of tumours. Patholgy and genetics of tumours of soft tissue and bone.2002.IARC PressLyon:
4. von Mehren M., Benjamin R., Bui M.M.: National Comprehensive Cancer Network (NCCN) clinical practice guidelines in oncology. Soft tissue sarcoma. Version 1.2013; Available at http://www.nccn.org/professionals/physician_gls/pdf/sarcoma.pdf Accessed October 19, 2013
5. Cheney M.D., Giraud C., Goldberg S.I., et. al.: MRI surveillance following treatment of extremity soft tissue sarcoma. J Surg Oncol 2014; 109: pp. 593-596.
6. Labarre D., Aziza R., Filleron T., et. al.: Detection of local recurrences of limb soft tissue sarcomas: is magnetic resonance imaging (MRI) relevant?. Eur J Radiol 2009; 72: pp. 50-53.
7. Rothermundt C., Whelan J.S., Dileo P., et. al.: What is the role of routine follow-up for localised limb soft tissue sarcomas? A retrospective analysis of 174 patients. Br J Cancer 2014; 110: pp. 2420-2426.
8. Watts A.C., Teoh K., Evans T., et. al.: MRI surveillance after resection for primary musculoskeletal sarcoma. J Bone Joint Surg Br 2008; 90: pp. 484-487.
9. Pisters P.W., Leung D.H., Woodruff J., et. al.: Analysis of prognostic factors in 1,041 patients with localized soft tissue sarcomas of the extremities. J Clin Oncol 1996; 14: pp. 1679-1689.
10. Coindre J.M., Terrier P., Bui N.B., et. al.: Prognostic factors in adult patients with locally controlled soft tissue sarcoma. A study of 546 patients from the French Federation of Cancer Centers Sarcoma Group. J Clin Oncol 1996; 14: pp. 869-877.
11. Stojadinovic A., Leung D.H., Allen P., et. al.: Primary adult soft tissue sarcoma: time-dependent influence of prognostic variables. J Clin Oncol 2002; 20: pp. 4344-4352.
12. Stojadinovic A., Leung D.H., Hoos A., et. al.: Analysis of the prognostic significance of microscopic margins in 2,084 localized primary adult soft tissue sarcomas. Ann Surg 2002; 235: pp. 424-434.
13. Vezeridis M.P., Moore R., Karakousis C.P.: Metastatic patterns in soft-tissue sarcomas. Arch Surg 1983; 118: pp. 915-918.
14. ESMO/European Sarcoma Network Working Group: Soft tissue and visceral sarcomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2012; 23: pp. vii92-vii99.
15. De Schepper A.M., De Beuckeleer L., Vandevenne J., et. al.: Magnetic resonance imaging of soft tissue tumors. Eur Radiol 2000; 10: pp. 213-223.
16. Varma D.G., Jackson E.F., Pollock R.E., et. al.: Soft-tissue sarcoma of the extremities. MR appearance of post-treatment changes and local recurrences. Magn Reson Imaging Clin N Am 1995; 3: pp. 695-712.
17. Kransdorf M.J., Murphey M.D.: Soft tissue tumors: post-treatment imaging. Radiol Clin North Am 2006; 44: pp. 463-472.
18. Garner H.W., Kransdorf M.J., Bancroft L.W., et. al.: Benign and malignant soft-tissue tumors: posttreatment MR imaging. Radiographics 2009; 29: pp. 119-134.
19. James S.L., Davies A.M.: Post-operative imaging of soft tissue sarcomas. Cancer Imaging 2008; 8: pp. 8-18.
20. Fitzgerald J., Roberts C.C., Daffner R.H., et. al.: ACR Appropriateness Criteria® follow-up of malignant or aggressive musculoskeletal tumors.2011.American College of RadiologyReston, VA Accessed November 24, 2013
21. Whooley B.P., Mooney M.M., Gibbs J.F., et. al.: Effective follow-up strategies in soft tissue sarcoma. Semin Surg Oncol 1999; 17: pp. 83-87.
22. Tseng W.W., Amini B., Madewell J.E.: Follow-up of the soft tissue sarcoma patient. J Surg Oncol 2015; 111: pp. 641-645.
23. Whooley B.P., Gibbs J.F., Mooney M.M., et. al.: Primary extremity sarcoma: what is the appropriate follow-up?. Ann Surg Oncol 2000; 7: pp. 9-14.
24. Al-Ibraheem A., Buck A.K., Benz M.R., et. al.: (18)F-Fluorodeoxyglucose positron emission tomography/computed tomography for the detection of recurrent bone and soft tissue sarcoma. Cancer 2013; 119: pp. 1227-1234.
25. Kole A.C., Nieweg O.E., van Ginkel R.J., et. al.: Detection of local recurrence of soft-tissue sarcoma with positron emission tomography using [18F]fluorodeoxyglucose. Ann Surg Oncol 1997; 4: pp. 57-63.
26. Partovi S., Chalian M., Fergus N., et. al.: Magnetic resonance/positron emission tomography (MR/PET) oncologic applications: bone and soft tissue sarcoma. Semin Roentgenol 2014; 49: pp. 345-352.
27. Del Grande F., Subhawong T., Weber K., et. al.: Detection of soft-tissue sarcoma recurrence: added value of functional MR imaging techniques at 3.0 T. Radiology 2014; 271: pp. 499-511.
28. Kransdorf M.J.: The use of gadolinium in the MR evaluation of musculoskeletal tumors. Top Magn Reson Imaging 1996; 8: pp. 15-23.
29. Vanel D., Shapeero L.G., Tardivon A., et. al.: Dynamic contrast-enhanced MRI with subtraction of aggressive soft tissue tumors after resection. Skeletal Radiol 1998; 27: pp. 505-510.
30. Vanel D., Lacombe M.J., Couanet D., et. al.: Musculoskeletal tumors: follow-up with MR imaging after treatment with surgery and radiation therapy. Radiology 1987; 164: pp. 243-245.
31. Robinson E., Bleakney R.R., Ferguson P.C., et. al.: Oncodiagnosis panel: 2007: multidisciplinary management of soft-tissue sarcoma. Radiographics 2008; 28: pp. 2069-2086.
32. Davidson A.C., Hinkley D.V.: Bootstrap methods and their application.1997.Cambridge University PressCambridge, UKpp. 594.