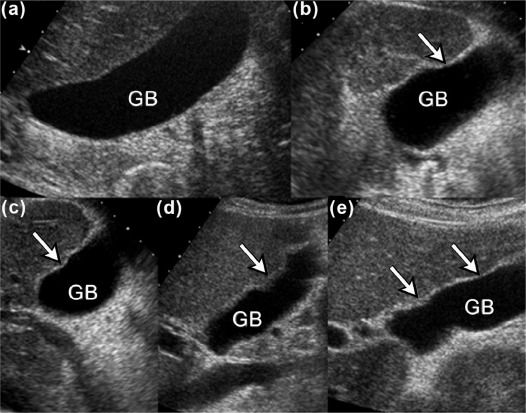
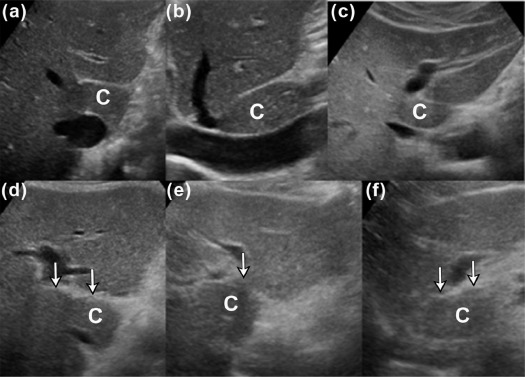
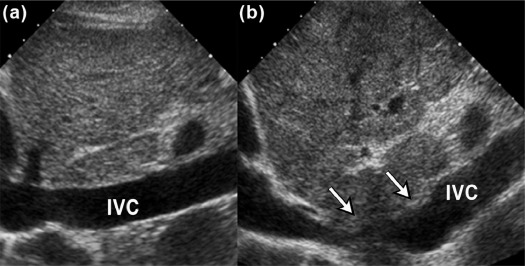
Purpose
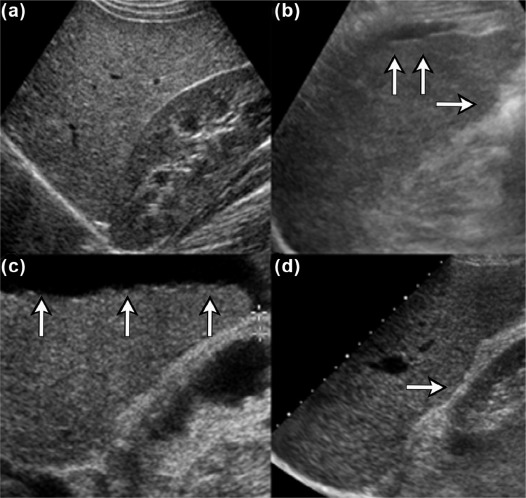
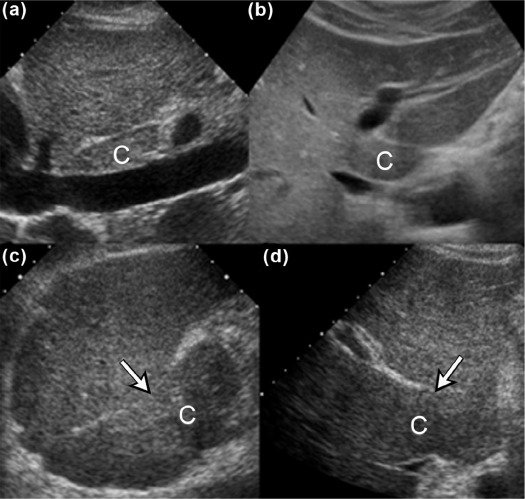
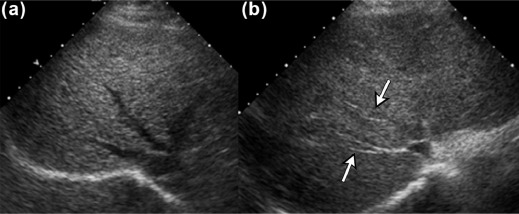
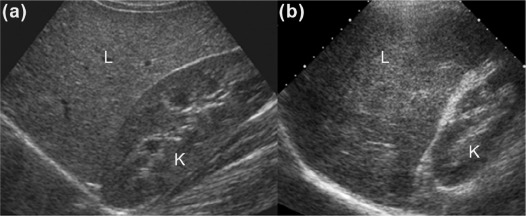
We aimed to present three new ultrasound signs—gallbladder scalloping, mammillated caudate lobe, and inferior vena cava scalloping—and determine their accuracy in diagnosing liver cirrhosis.
Materials and Methods
A total of 201 consecutive patients with a history of chronic liver disease who had undergone ultrasound imaging and liver biopsy were identified. A senior ultrasound radiologist blindly reviewed the ultrasound examinations. Specificity, sensitivity, positive predictive value, and negative predictive value of diagnosing cirrhosis were calculated for all evaluated ultrasound signs and selected combinations of signs, using the liver biopsy results as the reference standard.
Results
Of the 201 patients, 152 (76%) had either pathology-proven cirrhosis or significant fibrosis. Caudate lobe hypertrophy was the most specific (88%) and most positive predictor (90%) for cirrhosis, whereas mammillated caudate lobe was the most sensitive (78%). Inferior vena cava scalloping was the most specific (78%) of the three proposed ultrasound signs. When signs were combined, the presence of either gallbladder scalloping or liver surface nodularity was highly sensitive for cirrhosis (87%), whereas the presence of either gallbladder scalloping or inferior vena cava scalloping with caudate lobe hypertrophy was highly specific (93%).
Conclusions
Gallbladder scalloping, mammillated caudate lobe, and inferior vena cava scalloping are three novel signs that improve the accuracy of ultrasound in diagnosing cirrhosis.
Introduction
The diagnosis of cirrhosis using ultrasound (US) is difficult in patients with chronic liver disease. US imaging helps detect liver size and morphology, vascular flow patterns, and the presence or absence of ascites. It is widely accepted that liver surface nodularity and morphological changes such as caudate lobe hypertrophy, when severe enough, can be used to diagnose cirrhosis . Despite these findings, and the documented widespread use of US to screen for hepatocellular carcinoma , to this day, most physicians still rely on tissue biopsy for diagnosing cirrhosis.
Although perceived to be a definitive test, liver biopsy is not the ideal “gold standard.” Needle biopsy obtains a very small piece of tissue—approximately 1/50,000 of the entire liver—and is therefore subject to sampling error; false-negative rates of up to 30% have been reported . In addition, despite improvements in technique, biopsy remains an invasive procedure with a risk of complications, including bleeding, infection, and death . A need, therefore, exists for a reliable, noninvasive, rapid method for diagnosing liver fibrosis and cirrhosis . New techniques, including transient elastography, acoustic radiation force impulse, and magnetic resonance elastography have shown promising results. However, cost and operator inexperience have limited their widespread implementation .
Get Radiology Tree app to read full this article<
Materials and Methods
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Results
Get Radiology Tree app to read full this article<
TABLE 1
Diagnostic Performance of Known and Proposed Ultrasound Signs in 201 Patients with Chronic Liver Disease
Ultrasound Sign Sensitivity (%) Specificity (%) PPV (%) NPV (%) Known Caudate lobe hypertrophy 40 (32, 48) 88 (79, 97) 90 33 Hepatic vein compression 67 (60, 74) 72 (59, 85) 88 40 Coarse echotexture 57 (49, 65) 67 (54, 80) 84 34 Liver surface nodularity 74 (67, 81) 61 (47, 75) 85 43 Proposed GB scalloping 69 (62, 76) 62 (48, 76) 85 39 Mammillated caudate lobe 78 (71, 85) 60 (46, 74) 85 48 IVC scalloping 46 (38, 54) 78 (66, 90) 87 30
Values in parenthesis are 95% confidence intervals.
GB, gallbladder; IVC, inferior vena cava; NPV, negative predictive value; PPV, positive predictive value.
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
TABLE 2
Diagnostic Performance of Selected Known and Proposed Ultrasonographic Signs
Ultrasound Sign Sensitivity (%) Specificity (%) At least one present GB scalloping and caudate lobe hypertrophy 82 (76, 88) 54 (40, 68) GB scalloping and liver surface nodularity 87 (82, 92) 47 (33, 61) GB scalloping and mammillated caudate lobe 86 (80, 92) 39 (25, 53) Mammillated caudate lobe and caudate lobe hypertrophy 79 (73, 85) 60 (46, 74) Mammillated caudate lobe and hepatic vein compression 86 (80, 92) 46 (32, 60) Mammillated caudate lobe and liver surface nodularity 83 (77, 89) 46 (32, 60) Mammillated caudate lobe and IVC scalloping 82 (76, 88) 55 (41, 69) IVC scalloping and caudate lobe hypertrophy 64 (56, 72) 73 (61, 85) Both present GB scalloping and caudate lobe hypertrophy 27 (20, 34) 93 (86, 100) GB scalloping and liver surface nodularity 56 (48, 64) 74 (62, 86) GB scalloping and mammillated caudate lobe 62 (54, 70) 80 (69, 91) Mammillated caudate lobe and caudate lobe hypertrophy 39 (31, 47) 88 (79, 97) Mammillated caudate lobe and hepatic vein compression 56 (48, 64) 89 (80, 98) Mammillated caudate lobe and liver surface nodularity 69 (62, 76) 77 (65, 89) Mammillated caudate lobe and IVC scalloping 42 (34, 50) 85 (75, 95) IVC scalloping and caudate lobe hypertrophy 22 (15, 29) 93 (86, 100)
Values in parenthesis are 95% confidence intervals.
IVC, inferior vena cava; GB, gallbladder.
Get Radiology Tree app to read full this article<
Discussion
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Conclusion
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
References
1. Goyal N., Jain N., Rachapalli V., et. al.: Non-invasive evaluation of liver cirrhosis using ultrasound. Clin Radiol 2009; 64: pp. 1056-1066.
2. Irshad A., Anis M., Ackerman S.J.: Current role of ultrasound in chronic liver disease: surveillance, diagnosis and management of hepatic neoplasms. Curr Probl Diagn Radiol 2012; 41: pp. 43-51.
3. Afdhal N.H., Nunes D.: Evaluation of liver fibrosis: a concise review. Am J Gastroenterol 2004; 99: pp. 1160-1174.
4. Manning D.S., Afdhal N.H.: Diagnosis and quantitation of fibrosis. Gastroenterology 2008; 134: pp. 1670-1681.
5. Fontana R.J., Lok A.S.: Noninvasive monitoring of patients with chronic hepatitis C. Hepatology 2002; 36: pp. S57-S64.
6. Martinez S.M., Crespo G., Navasa M., et. al.: Noninvasive assessment of liver fibrosis. Hepatology 2011; 53: pp. 325-335.
7. Castera L.: Invasive and non-invasive methods for the assessment of fibrosis and disease progression in chronic liver disease. Best Pract Res Clin Gastroenterol 2011; 25: pp. 291-303.
8. American Institute of Ultrasound in Medicine : Practice guideline for the performance of an ultrasound examination of the abdomen and/or retroperitoneum. Available at http://www.aium.org/resources/guidelines.aspx
9. Brunt E.M.: Grading and staging the histopathological lesions of chronic hepatitis: the Knodell histology activity index and beyond. Hepatology 2000; 31: pp. 241-246.
10. Rumack C.M., Wilson S.R., Charboneau J.W., et. al.: Diagnostic ultrasound.4th ed.2011.Elsevier MosbySt. Louis
11. Weinstein S., Obuchowski N.A., Lieber M.L.: Clinical evaluation of diagnostic tests. AJR Am J Roentgenol 2005; 184: pp. 14-19.
12. Harbin W.P., Robert N.J., Ferrucci J.T.: Diagnosis of cirrhosis based on regional changes in hepatic morphology: a radiological and pathological analysis. Radiology 1980; 135: pp. 273-283.
13. Colli A., Fraquelli M., Andreoletti M., et. al.: Severe liver fibrosis or cirrhosis: accuracy of US for detection—analysis of 300 cases. Radiology 2003; 227: pp. 89-94.
14. Giorgio A., Amoroso P., Lettieri G., et. al.: Cirrhosis: value of caudate to right lobe ratio in diagnosis with US. Radiology 1986; 161: pp. 443-445.
15. Gaiani S., Gramantieri L., Venturoli N., et. al.: What is the criterion for differentiating chronic hepatitis from compensated cirrhosis? A prospective study comparing ultrasonography and percutaneous liver biopsy. J Hepatol 1997; 27: pp. 979-985.
16. Filly R.A., Reddy S.G., Nalbandian A.B., et. al.: Sonographic evaluation of liver nodularity: inspection of deep versus superficial surfaces of the liver. J Clin Ultrasound 2002; 30: pp. 399-407.
17. Di Lelio A., Cestari C., Lomazzi A., et. al.: Cirrhosis: diagnosis with sonographic study of the liver surface. Radiology 1989; 172: pp. 389-392.
18. Kitamura H., Kobayashi C.: Impairment of change in diameter of the hepatic portion of the inferior vena cava: a sonographic sign of liver fibrosis or cirrhosis. J Ultrasound Med 2005; 24: pp. 355-359. quiz 360-351
19. Khan M.H., Farrell G.C., Byth K., et. al.: Which patients with hepatitis C develop liver complications?. Hepatology 2000; 31: pp. 513-520.
20. Poynard T., Bedossa P., Opolon P.: Natural history of liver fibrosis progression in patients with chronic hepatitis C. The OBSVIRC, METAVIR, CLINIVIR, and DOSVIRC groups. Lancet 1997; 349: pp. 825-832.