Rationale and Objectives
The aims of this study were to evaluate the morphologic characteristics and growth pattern of hepatic tumors in H- ras 12V transgenic (TG) mice using a micro–magnetic resonance (MR) system and to assess the usefulness of gadolinium ethoxybenzyl diethylenetriamine penta-acetic acid (Gd-EOB-DTPA) enhancement for the detection of hepatic tumors in these mice.
Materials and Methods
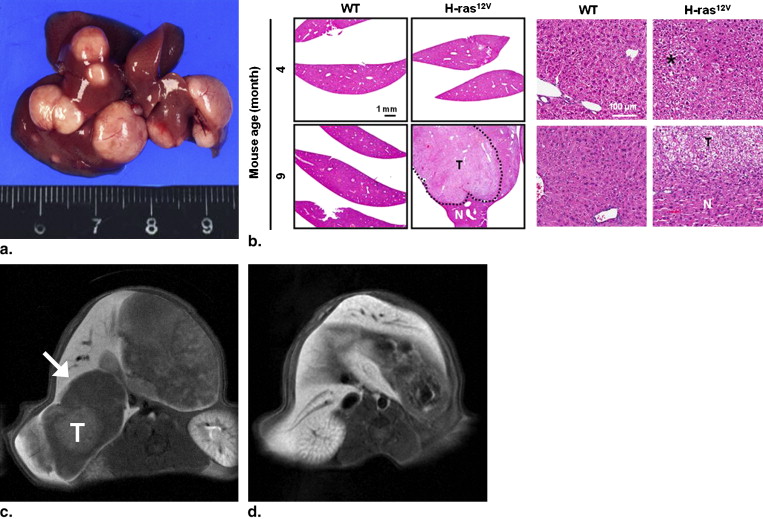
Hepatocellular carcinoma lines were established to allow insertion of the H- ras 12V transgene under the control of the albumin enhancer/promoter. Seven H- ras 12V TG mice and four wild-type mice were included in this study. The mice underwent various MR imaging examinations, including T1-weighted imaging (repetition time, 300 ms; echo time, 11 ms), Gd-EOB-DTPA-enhanced T1-weighted imaging (dose, 0.025 mmol/kg), and T2-weighted imaging (repetition time, 3500 ms; echo time, 36 ms), with a 4.7-T MR scanner, at 4, 6, 8, and 9 months of age. All mice were euthanized after the final MR imaging procedure, except for one TG mouse and two wild-type mice that were euthanized after MR imaging procedures at 4 months of age. For imaging analysis, the tumor characteristics in each MR sequence, including tumor size, number, and signal intensity (SI), were recorded, and the contrast-to-noise ratio and contrast enhancement ratio were calculated to quantify the SI of the tumor. The MR images were correlated with the findings of histopathologic examinations.
Results
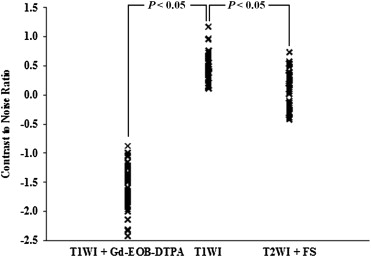
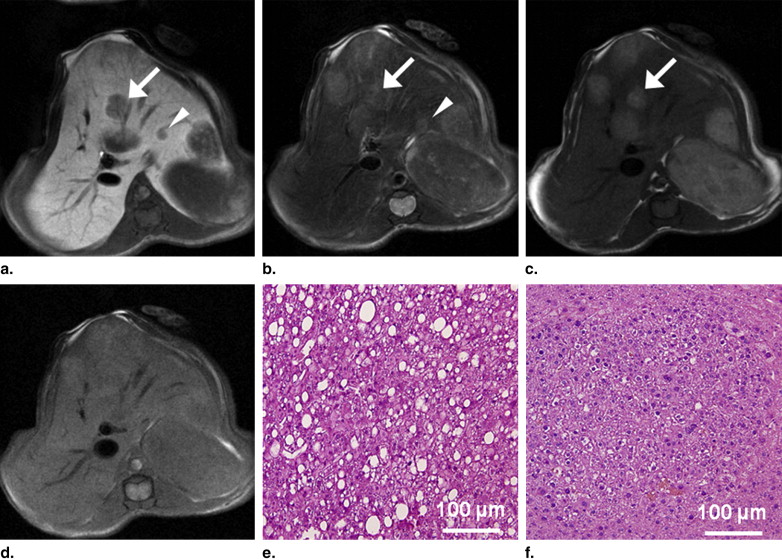
No tumors were detected in the four wild-type mice. In the six TG mice, a total of 67 tumors were found in histopathologic specimens obtained at 9 months of age. Of the 67 tumors, 62 were detected on Gd-EOB-DTPA-enhanced T1-weighted images with fat saturation. The majority of hepatic tumors showed high SI on T1-weighted images without fat saturation. The SI diminished on T1-weighted images with fat saturation. The tumor contrast-to-noise ratio for Gd-EOB-DTPA-enhanced T1-weighted imaging was significantly better than that for the other sequences. The tumors were histopathologically confirmed as hepatocellular adenomas ( n = 32) and well-differentiated hepatocellular carcinomas ( n = 35).
Conclusions
Micro-MR imaging can reveal the characteristics of hepatic tumors in a live murine model. Gd-EOB-DTPA-enhanced T1-weighted imaging is helpful in the detection of hepatic tumors in H- ras 12V TG mice.
Hepatocellular carcinoma (HCC) is a common malignancy in humans . Mutations of the ras gene have been detected in many human tumors. This gene encodes a small, signal-transducing guanosine triphosphatase that regulates the signaling pathways that control cell growth, differentiation, and survival . Although ras mutations are rare in human liver tumors , receptor-mediated hyperactivation of ras -dependent signal transduction pathways frequently occurs in human hepatocarcinogenesis . The three types of ras include N- ras , K- ras , and H- ras . Among these, murine HCCs express H- ras , which is activated in 70% of these cases; this finding suggests that H- ras plays a key role in hepatocarcinogenesis . Recently, H- ras transgenic (TG) mice were generated by using an H- ras 12V construct with a mouse albumin enhancer/promoter . The H- ras 12V TG mouse has 2 main advantages: (1) liver-specific expression and (2) long-term survival because of low expression levels of H- ras .
In vivo imaging technologies provide a unique opportunity for noninvasive and quantitative molecular-level analyses of diseases and for repeated and noninvasive monitoring of disease progression and/or response to treatment. The currently used small-animal model imaging technologies include magnetic resonance (MR), radionuclide imaging, computed tomography, ultrasonography, and optical imaging . Among these modalities, micro-MR imaging affords good microscopic resolution (micrometer-range resolution) and provides tissue-specific information when used with tissue-specific contrast agents . Tissue-specific liver MR imaging is useful for detecting and characterizing tumors. Hepatocyte-specific contrast agents are preferred over reticuloendothelial cell–specific contrast agents because the former offer superior tumor detection and lesion discrimination in murine hepatic tumors . In addition, the differences in the enhancement shown by hepatocyte-specific contrast agents can indicate additional tumor differentiation .
Get Radiology Tree app to read full this article<
Materials and methods
Generation of H- ras 12V TG Mice
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Animals
Get Radiology Tree app to read full this article<
MR Imaging
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Imaging Analysis and Statistical Analysis
Get Radiology Tree app to read full this article<
CNR=(SIofthetumor−SIoftheliver)/SIofbackmuscle, CNR
=
(
SI
of
the
tumor
−
SI
of
the
liver
)
/
SI
of
back
muscle
,
and
contrastenhancementratio=(SIonenhancedT1−weightedimaging−SIonnonenhancedT1−weightedimagingwithfatsaturation)/SIonnonenhancedT1−weightedimagingwithfatsaturation. contrast
enhancement
ratio
=
(
SI
on
enhanced
T
1
-
weighted
imaging
-
SI
on
nonenhanced
T
1
-
weighted
imaging
with
fat
saturation
)
/
SI
on
nonenhanced
T
1
-
weighted
imaging
with
fat
saturation
.
On an enhanced image, a CNR value < 0 indicates negative contrast enhancement, and a CNR value > 0 indicates positive contrast enhancement.
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Histopathology
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Results
MR Imaging
Number and Sizes of Tumors Depicted on MR Images
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Table 1
Mean Sizes (mm) of Hepatic Tumors in H- ras 12V Transgenic Mice on Magnetic Resonance Images
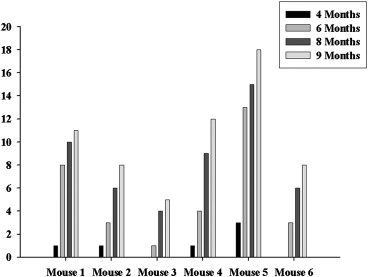
Age (months) Mouse 4 6 8 9 1 1.20 1.80 ± 0.81 5.45 ± 3.28 6.81 ± 3.89 2 0.60 2.10 ± 1.32 4.43 ± 3.02 4.78 ± 2.45 3 — 2.10 4.45 ± 2.55 4.80 ± 3.44 4 1.10 2.13 ± 1.27 4.44 ± 1.95 4.28 ± 2.79 5 1.20 ± 0.17 2.43 ± 1.17 4.10 ± 2.16 4.36 ± 2.71 6 — 1.60 ± 0.36 3.58 ± 2.43 3.76 ± 2.96 Mean 1.08 ± 0.26 2.11 ± 1.02 4.44 ± 2.48 4.79 ± 3.06
Data are expressed as mean ± SD.
Get Radiology Tree app to read full this article<
Morphologic Characteristics Depicted on MR Images
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Morphologic Changes Depicted on MR Images
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Histopathology-MR Correlation
Number and Sizes of Tumors
Get Radiology Tree app to read full this article<
Table 2
Detectability of Hepatic Tumors in H- ras 12V Transgenic Mice on Magnetic Resonance Images
Number of Tumors (Detectability) Tumor Size (mm) Histopathology T1WIs without Fat Saturation T2WIs with Fat Saturation Gd-EOD-DTPA–Enhanced T1WIs >2 50 49 (98.0%) 45 (90.0%) 50 (100%) 0.4–2 17 3 (17.6%) 1 (5.9%) 12 (70.6%) Total 67 52 (77.6%) 46 (68.7%) 62 (92.5%)
Gd-EOB-DTPA, gadolinium ethoxybenzyl diethylenetriamine penta-acetic acid; T1WI, T1-weighted image; T2WI, T2-weighted image.
Get Radiology Tree app to read full this article<
Morphological Characteristics of Tumors
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Discussion
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Conclusions
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
References
1. Parkin D.M., Bray F., Ferlay J., et. al.: Global cancer statistics. CA Cancer J Clin 2005; 55: pp. 74-108.
2. Sherman M.: Hepatocellular carcinoma: epidemiology, risk factors, and screening. Semin Liver Dis 2005; 25: pp. 143-154.
3. Bos J.L.: Ras oncogenes in human cancer; a review. Cancer Res 1989; 49: pp. 4682-4689.
4. Boguski M.S., McCormick F.: Proteins regulating Ras and its relatives. Nature 1993; 366: pp. 643-654.
5. Tada M., Omata M., Ohto M.: Analysis of ras gene mutations in human hepatic malignant tumors by polymerase chain reaction and direct sequencing. Cancer Res 1990; 50: pp. 1121-1124.
6. Kida T., Tsuda H., Pairojkul C., et. al.: Mutations of the p53 tumor suppressor gene and the ras gene family in intrahepatic cholangiocellular carcinomas in Japan and Thailand. Mol Carcinog 1993; 8: pp. 312-318.
7. Staib F., Hussain S.P., Hofseth L.F., et. al.: TP53 and liver carcinogenesis. Hum Mutat 2003; 21: pp. 201-216.
8. Gotzmann J., Huber H., Thallinger C., et. al.: Hepatocytes convert to a fibroblastoid phenotype through the cooperation of TGF-beta1 and Ha-Ras: steps towards invasiveness. J Cell Sci 2002; 115: pp. 1189-1202.
9. Frith C.H., Ward J.M.: A morphologic classification of proliferative and neoplastic hepatic lesions in mice. J Environ Pathol Toxicol 1979; 3: pp. 329-351.
10. Reynolds S.H., Stowers S.J., Maronpot R.R., et. al.: Detection and identification of activated oncogenes in spontaneously occurring benign and malignant hepatocellular tumors of the B6C3F1 mouse. Proc Natl Acad Sci U S A 1986; 83: pp. 33-37.
11. Gilbert E., Morel A., Tulliez M., et. al.: In vivo effects of activated H-ras oncogene expressed in the liver and in urogenital tissues. Int J Cancer 1997; 73: pp. 749-756.
12. Hayashi S., Mori I., Nonoyama T., et. al.: Point mutation of the c-H-ras gene in spontaneous liver tumors of transgenic mice carrying the human c-H-ras gene. Toxicol Pathol 1998; 28: pp. 556-561.
13. Sandgren E.P., Quaife C.J., Pinkert C.A., et. al.: Oncogene-induced liver neoplasia in transgenic mice. Oncogene 1989; 4: pp. 715-724.
14. Tremblay P.J., Pothier F., Hoang T., et. al.: Transgenic carrying the mouse mammary tumor virus ras fusion gene: distinct effects in various tissues. Mol Cell Biol 1989; 9: pp. 854-859.
15. Wang A.G., Moon H.B., Lee M.R., et. al.: Gender-dependent hepatic alterations in H-ras12V transgenic mice. J Hepatol 2005; 43: pp. 836-844.
16. Allport J.R., Weissleder R.: In vivo imaging of gene and cell therapies. Exp Hematol 2001; 29: pp. 1237-1246.
17. Bulte J.W., Kraitchman D.L.: Iron oxide MR contrast agents for molecular and cellular imaging. NMR Biomed 2004; 17: pp. 484-499.
18. Fujata M., Yamamoto R., Fritz-Zieroth B., et. al.: Contrast enhancement with Gd-EOB-DTPA in MR imaging of hepatocellular carcinoma in mice: a comparison with superparamagnetic iron oxide. J Magn Reson Imaging 1996; 6: pp. 472-477.
19. Ni Y., Marchal G., Yu J., et. al.: Prolonged positive contrast enhancement with Gd-EOB-DTPA in experimental liver tumors: potential value in tissue characterization. J Magn Reson Imaging 1994; 4: pp. 355-363.
20. Moon E.Y., Lee M.R., Wang A.G., et. al.: Delayed occurrence of H-ras12V-induced hepatocellular carcinoma with long-term treatment with cinnamaldehydes. Eur J Pharmacol 2006; 530: pp. 270-275.
21. Kiryu S., Inoue Y., Watanabe M., et. al.: Evaluation of gadoxetate disodium as a contrast agent for mouse liver imaging: comparison with gadobenate dimeglumine. Magn Reson Imaging 2009; 27: pp. 101-107.
22. Pearline R.V., Lin Y., Shen K.J., et. al.: Alterations in enzymatic functions in hepatocytes and hepatocellular carcinomas from Ras-transduced livers resemble the effects of insulin. Hepatology 1996; 24: pp. 838-848.
23. Eguchi A., Nakashima O., Okudaira S., et. al.: Adenomatous hyperplasia in the vicinity of small hepatocellular carcinoma. Hepatology 1992; 15: pp. 843-848.
24. Nakanuma Y., Terada T., Ueda K., et. al.: Adenomatous hyperplasia of the liver as a precancerous lesion. Liver 1993; 13: pp. 1-9.
25. Terada T., Nakanuma Y., Hoso M., et. al.: Fatty macro-regenerative nodule in non-steatotic liver cirrhosis: a morphologic study. Virchows Arch A Pathol Anat Histopathol 1989; 415: pp. 131-136.
26. Winter T.C., Takayasu K., Muramatsu Y., et. al.: Early advanced hepatocellular carcinoma: evaluation of CT and MR appearance with pathologic correlation. Radiology 1994; 192: pp. 379-387.
27. Kim J.I., Lee J.M., Choi J.Y., et. al.: The value of gadobenate dimeglumine-enhanced delayed phase MR imaging for characterization of hepatocellular nodules in the cirrhotic liver. Invest Radiol 2008; 43: pp. 202-210.
28. Narita M., Hatano E., Arizono S., et. al.: Expression of OATP1B3 determines uptake of Gd-EOB-DTPA in hepatocellular carcinoma. J Gastroenterol 2009; 44: pp. 793-798.