Rationale and Objectives
Blood perfusion of peripheral nerves plays an important role in regeneration after nerve injury. Functional recovery after a peripheral nerve injury depends not only on the survival of the affected neurons but also on the recovered blood perfusion. Previous studies have shown that it is possible to quantitatively assess blood perfusion of tissue using contrast-enhanced ultrasound (CEUS). The aim of this study was to evaluate the usefulness of CEUS for the quantitative evaluation of blood perfusion of the sciatic nerves with crush injury.
Materials and Methods
Crush injuries were created in the left sciatic nerve of 30 New Zealand white rabbits. CEUS of the bilateral sciatic nerves was performed in six experimental rabbits at 3 days, 1 week, 2 weeks, 4 weeks, and 8 weeks after injury. Pulse-inversion harmonic imaging was used for real-time CEUS. The other six rabbits were used as a control group. Serial laser Doppler measurements of blood flow and quantitative histologic evaluation were performed parallel to CEUS on all animals.
Results
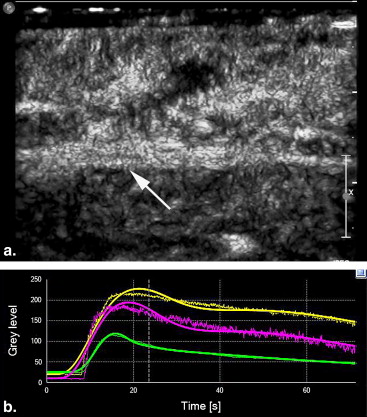
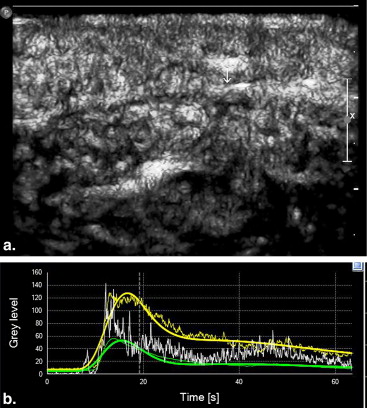
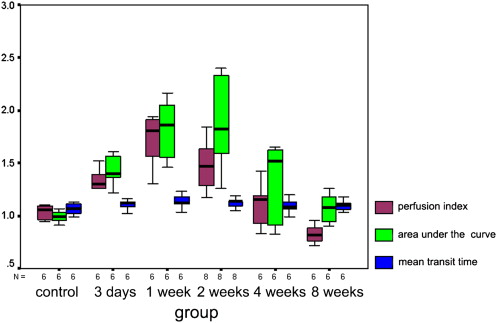
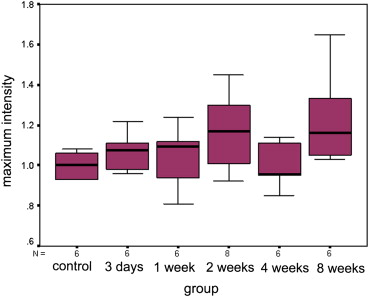
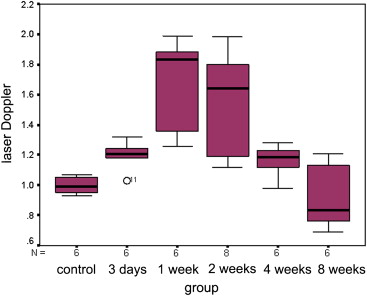
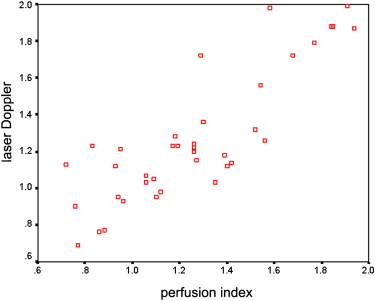
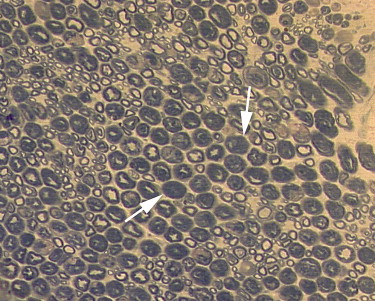
Quantitative analysis of CEUS showed that the perfusion index of the crushed sciatic nerves was increased at 3 days after injury, with a peak at 1 week after injury ( P = .000). The area under the curve for the crushed sites was increased at 3 days after injury, with a peak at 2 weeks after injury ( P = .000). The mean transit time and maximum intensity of the crushed site of the left sciatic nerves were not significantly changed during the 2 months after injury ( P = .335 and P = .157 respectively). The perfusion indices measured by CEUS correlated well with those measured by laser Doppler ( r = 0.791, P = .000). Marked Wallerian degeneration was found at the crushed site of sciatic nerves at 3 days after injury. The percentage of degenerated myelinated axons was increased during the first 2 weeks after injury and then decreased during the following period. Regenerated axons with small diameter and thin myelin sheaths were found at 2 weeks after injury and during the following period.
Conclusions
CEUS may provide a new imaging method to quantitatively analyze blood perfusion of injured peripheral nerves.
The assessment of blood perfusion of peripheral nerves is of great value in clinical practice because of its close relationship with axonal regeneration. Functional recovery after a peripheral nerve injury depends not only on the survival of the affected neurons but also on the recovered blood perfusion. Nerve ischemia may occur because of thrombosis, endothelial swelling, endoneurial edema, granulocyte plugging of the vasa nervorum, or actual physical interruption of microvessels at the site of injury . With regard to nerve grafts, vascularized nerve grafts have been proved superior to nonvascularized nerve grafts with respect to healing, especially when used in the hypovascular and scarred recipient bed . Because of the presence of sufficient and uninterrupted blood supply to the nerve graft, Schwann cell viability could be high, which would result in successful nerve healing. Thus, therapies that could promote the angiogenesis of peripheral nerves, such as cytokines, genes, or increasing blood flow, have been tried, and the results have shown that an increase in posttraumatic nerve blood flow can significantly improve the function of injured axons .
The current techniques that have been used for evaluating peripheral nerve blood perfusion include radioactive microspheres, microelectrode hydrogen clearance polarography, laser Doppler flowmetry, and others . All these methods are unsuitable for the noninvasive assessment of blood perfusion in peripheral nerves in clinical practice. Contrast-enhanced ultrasound (CEUS), which is based on the use of gas-filled microbubbles, can display blood perfusion at the capillary level . Because these microbubbles have a microvascular rheology similar to that of red blood cells and remain entirely within the vascular space, they reflect blood volume more accurately than computed tomographic and magnetic resonance imaging contrast agents, which extravasate into the interstitial space . Despite these promising results of CEUS in evaluating tissue perfusion, few studies have been performed using CEUS to evaluate blood perfusion of the peripheral nerves. Our previous study showed that it was possible to quantitatively evaluate blood flow of the sciatic nerve in normal New Zealand white rabbits . Thus, we hypothesized that CEUS could be used to noninvasively assess blood perfusion of injured peripheral nerves. To test our hypothesis, CEUS of sciatic nerves with a crush injury model in New Zealand white rabbits was performed and compared to data on blood perfusion measured using laser Doppler and to axonal changes on histopathology.
Materials and methods
Animals and Surgical Procedure
Get Radiology Tree app to read full this article<
Sonographic Contrast Agent
Get Radiology Tree app to read full this article<
Sonographic Equipment and CEUS Examination
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Offline Analysis of CEUS
Get Radiology Tree app to read full this article<
Perfusion Measurement with Laser Doppler
Get Radiology Tree app to read full this article<
Histopathologic Examination
Get Radiology Tree app to read full this article<
Statistical Analysis
Get Radiology Tree app to read full this article<
Results
CEUS
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Laser Doppler Blood Perfusion Measurement
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Histopathologic Examination
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Discussion
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Conclusions
Get Radiology Tree app to read full this article<
References
1. Lundborg G., Myers R., Powell H.: Nerve compression injury and increased endoneurial fluid pressure: a “miniature compartment syndrome”. J Neurol Neurosurg Psychiatry 1983; 46: pp. 1119-1124.
2. Schmelzer J.D., Zochodne D.W., Low P.A.: Ischemic and reperfusion injury of rat peripheral nerve. Proc Natl Acad Sci USA 1989; 86: pp. 1639-1642.
3. Schmid-Schonbein G.W.: Capillary plugging by granulocytes and no-reflow phenomenon in the microcirculation. Fed Proc 1987; 46: pp. 2397-2401.
4. Vargel I., Demirci M., Erdem S., et. al.: A comparison of various vascularization-perfusion venous nerve grafts with conventional nerve grafts in rats. J Reconstr Microsurg 2009; 25: pp. 425-437.
5. Vargel I.: Impact of vascularization type on peripheral nerve microstructure. J Reconstr Microsurg 2009; 25: pp. 243-253.
6. Rovak J.M., Mungara A.K., Aydin M.A., et. al.: Effects of vascular endothelial growth factor on nerve regeneration in acellular nerve grafts. J Reconstr Microsurg 2004; 20: pp. 53-58.
7. Pan H.C., Wu H.T., Cheng F.C., et. al.: Potentiation of angiogenesis and regeneration by G-CSF after sciatic nerve crush injury. Biochem Biophys Res Commun 2009; 382: pp. 177-182.
8. Fehlings M.G., Tator C.H., Linden R.D.: The effect of nimodipine and dextran on axonal function and blood flow following experimental spinal cord injury. J Neurosurg 1989; 71: pp. 403-416.
9. Maki Y., Firrell J.C., Breidenbach W.C.: Blood flow in mobilized nerves: results in a rabbit sciatic nerve model. Plast Reconstr Surg 1997; 100: pp. 627-633.
10. Höke A., Sun H.S., Gordon T., et. al.: Do denervated peripheral nerve trunks become ischemic? The impact of chronic denervation on vasa nervorum. Exp Neurol 2001; 172: pp. 398-406.
11. Jou I.M., Lai K.A., Shen C.L., et. al.: Changes in conduction, blood flow, histology, and neurological status following acute nerve-stretch injury induced by femoral lengthening. J Orthop Res 2000; 18: pp. 149-155.
12. Zhang W.Z., Zha D.G., Cheng G.X., et. al.: Assessment of regional myocardial blood flow with myocardial contrast echocardiography: an experimental study. Echocardiography 2004; 21: pp. 409-416.
13. Kalantarinia K., Belcik J.T., Patrie J.T., et. al.: Real-time measurement of renal blood flow in healthy subjects using contrast-enhanced ultrasound. Am J Physiol Renal Physiol 2009; 297: pp. F1129-F1134.
14. Lindner J.R., Song J., Jayaweera A.R., et. al.: Microvascular rheology of Definity microbubbles after intra-arterial and intravenous administration. J Am Soc Echocardiogr 2002; 15: pp. 396-403.
15. Wang Y., Tang P., Zhang L., et. al.: Quantitative evaluation of the peripheral nerve blood perfusion with high frequency contrast-enhanced ultrasound. Acad Radiol 2010; 17: pp. 1492-1497.
16. Giorgio A., De Stefano G., Coppola C., et. al.: Contrast-enhanced sonography in the characterization of small hepatocellular carcinomas in cirrhotic patients: comparison with contrast-enhanced ultrafast magnetic resonance imaging. Anticancer Res 2007; 27: pp. 4263-4269.
17. Lu M.D., Yu X.L., Li A.H., et. al.: Comparison of contrast enhanced ultrasound and contrast enhanced CT or MRI in monitoring subcutaneous thermal ablation procedure in patients with hepatocellular carcinoma: a multi-center study in China. Ultrasound Med Biol 2007; 33: pp. 1736-1749.
18. Cosgrove D., Eckersley R., Blomley M., et. al.: Quantification of blood flow. Eur Radiol 2001; 11: pp. 1338-1344.
19. Thijssen J.M., de Korte C.L.: Modeling ultrasound contrast measurement of blood flow and perfusion in biological tissue. Ultrasound Med Biol 2005; 31: pp. 279-285.
20. Rissanen T.T., Korpisalo P., Karvinen H., et. al.: High-resolution ultrasound perfusion imaging of therapeutic angiogenesis. JACC Cardiovasc Imaging 2008; 1: pp. 83-91.
21. Yoshizawa H., Kobayashi S., Kubota K.: Effects of compression on intraradiular blood flow in dogs. Spine 1989; 14: pp. 1220-1225.
22. Olmarker K., Holm S., Rosenqvist A.L., et. al.: Experimental nerve root compression: a model of acute, graded compression of the porcine cauda equina and an analysis of neural and vascular anatomy. Spine 1991; 16: pp. 61-69.
23. Rydevik B., Lundborg G., Bagge U.: Effects of graded compression on intraneural blood flow, an in vivo study on rabbit tibial nerve. J Hand Surg Am 1981; 6: pp. 3-12.
24. Zochodne D.W., Ho L.T.: Endoneurial microenvironment and acute nerve crush injury in the rat sciatic nerve. Brain Research 1990; 535: pp. 43-48.
25. Zochodne D.W., Allison J.A., Ho W., et. al.: Evidence for CGRP accumulation and activity in experimental neuromas. Am J Physiol 1995; 268: pp. H584-H590.
26. Zochodne D.W., Nguyen C., Sharkey K.A.: Accumulation and degranulation of mast cells in experimental neuromas. Neurosci Lett 1994; 182: pp. 3-6.
27. Fehlings M.G., Tator C.H., Linden R.D.: The relationships among the severity of spinal cord injury, motor and somatosensory evoked potentials and spinal cord blood flow. Electroencephalogr Clin Neurophysiol 1989; 74: pp. 241-259.
28. Zochodne D.W., Nguyen C.: Angiogenesis at the site of neuroma formation in transected peripheral nerve. J Anat 1997; 191: pp. 23-30.
29. Lindholm D., Heumann R., Meyer M., et. al.: Interleukin-1 regulates synthesis of nerve growth factor in non-neuronal cells of rat sciatic nerve. Nature 1987; 330: pp. 658-659.
30. Degeorges R., Lebellec Y., Alnot J.Y.: Prognostic factors of axillary nerve surgery. Rev Chir Orthop Reparatrice Appar Mot 2004; 90: pp. 103-110.
31. Wehbe J., Maalouf G., Habanbo J., et. al.: Surgical treatment of traumatic lesions of the axillary nerve. A retrospective study of 33 cases. Acta Orthop Belg 2004; 70: pp. 11-18.
32. Du J., Li F.H., Fang H., et. al.: Correlation of real-time gray scale contrast-enhanced ultrasonography with microvessel density and vascular endothelial growth factor expression for assessment of angiogenesis in breast lesions. J Ultrasound Med 2008; 27: pp. 821-831.
33. Krix M., Kiessling F., Vosseler S., et. al.: Comparison of intermittent-bolus contrast imaging with conventional power Doppler sonography: quantification of tumour perfusion in small animals. Ultrasound Med Biol 2003; 29: pp. 1093-1103.
34. Wang Z., Tang J., An L., et. al.: Contrast-enhanced ultrasonography for assessment of tumor vascularity in hepatocellular carcinoma. J Ultrasound Med 2007; 26: pp. 757-762.
35. Klauser A., Demharter J., De Marchi A., et. al.: Contrast-enhanced gray-scale sonography in assessment of joint vascularity in rheumatoid arthritis: results from the IACUS study group. Eur Radiol 2005; 15: pp. 2404-2410.