Rationale and Objectives
Previous studies showed it was possible to employ sonographic contrast agent for identification of the sentinel lymph nodes (SLNs). This study is to investigate the usefulness of SonoVue (a sonographic contrast agent) and gray-scale contrast-enhanced ultrasonography (CEUS) for detecting the SLNs in a metastatic breast cancer model.
Materials and Methods
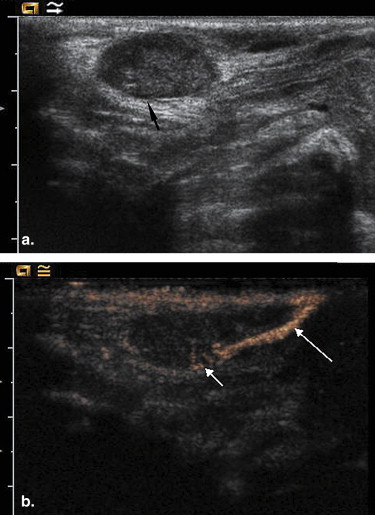
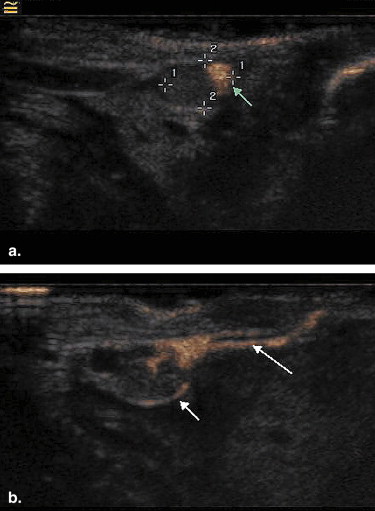
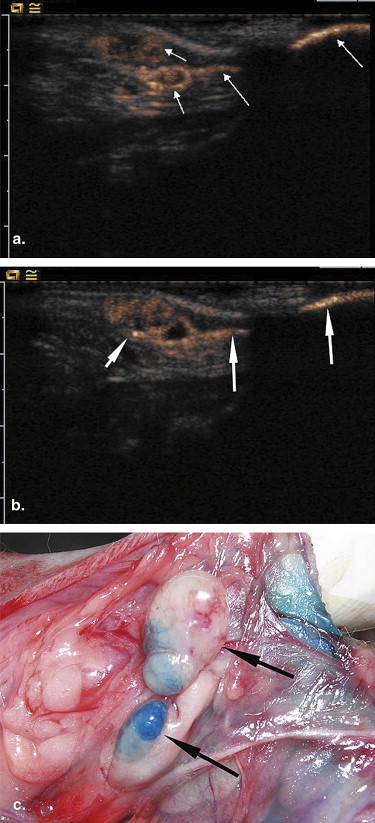
CEUS was performed in 12 female rabbits with breast VX2 tumor after subcutaneous administration of SonoVue. The site, number, and pattern of enhancement of the SLNs were observed and recorded. After CEUS, 0.5 mL of blue dye was injected into the same location as SonoVue and the SLNs were detected by surgical dissection. The findings of CEUS were compared with those of blue dye.
Results
Of the 12 tumors assessed, a total of 17 enhanced SLNs were detected by CEUS. Among them, a single SLN was detected in eight tumors, two SLNs in three tumors, and three SLNs in one tumor. All the SLNs showed partial enhancement on CEUS. Nineteen SLNs were identified by blue dye with surgical dissection. There were no false-positive CEUS findings in terms of SLN detection. The overall sensitivity of CEUS for detecting SLNs was 89.5% (17/19). Among the 17 SLNs detected by CEUS, tumor metastases were identified histopathologically in 4 SLNs, whereas proliferation of lymphatic tissue was identified in the other 13 SLNs.
Conclusions
CEUS combined with SonoVue is useful for detecting SLNs, although it may not be helpful for detecting metastases in SLNs.
The sentinel lymph node (SLN) is the first node (or nodes) in the regional lymph node drainage basin to receive afferent lymphatic drainage from the primary tumor . During the past decade, sentinel lymph node biopsy has been proved a useful method to determine the lymph node status in patients with a variety of tumors, ranging from head-and-neck melanoma, breast carcinoma, to vulvar carcinoma . In patients with early-stage breast cancer, sentinel lymph node biopsy is standard care and axillary lymph node dissection is considered unnecessary when SLNs are tumor-free . Additional non-SLN metastasis in patients with positive SLNs can be estimated using several risk factors such as primary tumor size, metastatic tumor size in SLNs, lymphatic vessel invasion, and so on. All patients with positive SLNs may be treated with further axillary lymph node dissection based on their own risk for non-SLN metastasis. Thus, it is essential to detect accurately the SLNs when performing the sentinel lymph node biopsy.
Up to now, two methods for detection of SLNs have been routinely used either independently or together. First, the use of vital blue dyes, which provide visual identification of draining lymphatic channels (LCs), as well as SLNs after dissection . Second, the use of radiopharmaceuticals, which can be detected either before surgery with a gamma camera or intraoperatively with radioactive sensor probes or Geiger counters . Although the accuracy of these established approaches is high, they have potential limitations and adverse effects. For instance, the use of blue dye to identify SLNs requires surgical dissection that, in some cases, can be extensive, and there is a danger of anaphylactic reactions . Lymphoscintigraphy requires the use of radiation; thus, there is exposure to the patient as well as the surgeon and support personnel. Additionally, when lymphoscintigraphy is used, SLNs that are outside the imaging field or behind one another may not be adequately detected, leading to an incomplete SLN detection. Because most radioactive materials make use of small particles such as human serum albumin and colloid albumin, as well as filtered sulfur colloids, these small particles may pass through the SLNs, resulting in identification of secondary lymph nodes (ie, nodes that are not SLNs) .
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Materials and methods
Animal Model
Get Radiology Tree app to read full this article<
Sonographic Contrast Agent
Get Radiology Tree app to read full this article<
Sonographic Equipment
Get Radiology Tree app to read full this article<
Gray-scale CEUS
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Blue Dye and Histopathologic Examinations
Get Radiology Tree app to read full this article<
Results
Animals and Implanted Tumors
Get Radiology Tree app to read full this article<
Gray-scale CEUS
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Blue Dye Method and Pathology
Get Radiology Tree app to read full this article<
Discussion
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
References
1. Yudd A.P., Kempf J.S., Goydos J.S., et. al.: Use of sentinel node lymphoscintigraphy in malignant melanoma. Radiographics 1999; 19: pp. 343-353.
2. Minamikawa T., Umeda M., Komori T.: Reliability of sentinel lymph node biopsy with squamous cell carcinoma of the oral cavity. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2005; 99: pp. 532-538.
3. Burak W.E., Hollenbeck S.T., Zervos E.E., et. al.: Sentinel lymph node biopsy results in less postoperative morbidity compared with axillary lymph node dissection for breast cancer. Am J Surg 2002; 183: pp. 23-27.
4. de Hullu J.A., Hollema H., Piers D.A., et. al.: Sentinel lymph node procedure is highly accurate in squamous cell carcinoma of the vulva. J Clin Oncol 2000; 18: pp. 2811-2816.
5. Wright B.E., Scheri R.P., Ye X., et. al.: Importance of sentinel lymph node biopsy in patients with thin melanoma. Arch Surg 2008; 143: pp. 892-899.
6. Veronesi U., Paganelli G., Viale G., et. al.: A randomized comparison of sentinel-node biopsy with routine axillary dissection in breast cancer. N Engl J Med 2003; 349: pp. 546-553.
7. Bostick P.J., Giuliano A.E.: Vital dyes in sentinel node localization. Semin Nucl Med 2000; 30: pp. 18-24.
8. Meyer-Rochow G.Y., Martin R.C., Harman C.R.: Sentinel node biopsy in breast cancer: validation study and comparison of blue dye alone with triple modality localization. ANZ J Surg 2003; 73: pp. 815-818.
9. Haigh P.I., Glass E.C., Essner R.: Accuracy of gamma probes in localizing radioactivity: in-vitro assessment and clinical implications. Cancer Biother Radiopharm 2000; 15: pp. 561-569.
10. Glass E.C., Essner R., Morton D.L.: Kinetics of three lymphoscintigraphic agents in patients with cutaneous melanoma. J Nucl Med 1998; 39: pp. 1185-1190.
11. Gimenez J., Botella-Estrada R., Hernandez D., et. al.: Anaphylaxis after peritumoral injection of sulfan blue 1% for identification of the sentinel lymph node in lymphatic mapping of the breast: case report. Eur J Surg 2001; 167: pp. 921-923.
12. Goldfarb L.R., Alazraki N., Eshima D., et. al.: Lymphoscintigraphic identification of sentinel lymph nodes: clinical evaluation of 0.22 mm filtration of Tc-99m sulfur colloid. Radiology 1998; 208: pp. 505-509.
13. Mattrey R.F., Kono Y., Baker K., et. al.: Sentinel lymph node imaging with microbubble ultrasound contrast material. Acad Radiol 2002; 9: pp. S231-S235.
14. Wisner E.R., Ferrara K.W., Short R.E., et. al.: Sentinel node detection using contrast-enhanced power Doppler ultrasound lymphography. Invest Radiol 2003; 38: pp. 358-365.
15. Goldberg B.B., Merton D.A., Liu J.B., et. al.: Sentinel lymph nodes in a swine model with melanoma: contrast-enhanced lymphatic US. Radiology 2004; 230: pp. 727-734.
16. Goldberg B.B., Merton D.A., Liu J.B., et. al.: Contrast-enhanced sonographic imaging of lymphatic channels and sentinel lymph nodes. J Ultrasound Med 2005; 24: pp. 953-965.
17. Choi S.H., Kono Y., Corberi J., et. al.: Model to quantify lymph node enhancement on indirect sonographic lymphography. Am J Roentgenol 2004; 183: pp. 513-517.
18. Lurie D.M., Seguin B., Schneider P.D., et. al.: Contrast-assisted ultrasound for sentinel lymph node detection in spontaneously arising canine head and neck tumors. Invest Radiol 2006; 41: pp. 415-421.
19. Omoto K., Hozumi Y., Omoto Y., et. al.: Sentinel node detection in breast cancer using contrast-enhanced sonography with 25% albumin—initial clinical experience. J Clin Ultrasound 2006; 34: pp. 317-326.
20. Hanna G., Hopkins R., Flaim K., et. al.: Indirect lymphography with perflubron emulsion. Preclinical and clinical results. Invest Radiol 1994; 29: pp. S33-S35.
21. Giorgio A., De Stefano G., Coppola C., et. al.: Contrast-enhanced sonography in the characterization of small hepatocellular carcinomas in cirrhotic patients: comparison with contrast-enhanced ultrafast magnetic resonance imaging. Anticancer Res 2007; 27: pp. 4263-4269.
22. Lu M.D., Yu X.L., Li A.H., et. al.: Comparison of contrast enhanced ultrasound and contrast enhanced CT or MRI in monitoring subcutaneous thermal ablation procedure in patients with hepatocellular carcinoma: a multi-center study in China. Ultrasound Med Biol 2007; 33: pp. 1736-1749.
23. Chen J.H., Ling R., Yao Q., et. al.: Effect of small-sized liposomal Adriamycin administered by various routes on a metastatic breast cancer model. Endocrine-related Cancer 2005; 12: pp. 93-100.
24. Gorce J.M., Arditi M., Schneider M.: Influence of bubble size distribution on the echogenicity of ultrasound contrast agents: a study of SonoVue. Invest Radiol 2000; 35: pp. 661-671.
25. Needleman L., Blomley M.J., Albrecht T., et. al.: Reticuloendothelial system-specific phase Sonazoid-enhanced ultrasound: results of an exploratory multi-center study for liver lesion detection. [abstract] Radiology 2002; 225: pp. 247.
26. Watanabe R., Matsumura M., Chen C.J., et. al.: Characterization of tumor imaging with microbubble-based ultrasound contrast agent, Sonazoid, in rabbit liver. Biol Pharm Bull 2005; 28: pp. 972-977.
27. Kindberg G.M., Tolleshaug H., Roos N., et. al.: Hepatic clearance of Sonazoid perfluorobutane microbubbles by Kupffer cells does not reduce the ability of liver to phagocytose or degrade albumin microspheres. Cell Tissue Res 2003; 312: pp. 49-54.
28. Watanabe R., Matsumura M., Munemasa T., et. al.: Mechanism of hepatic parenchyma-specific contrast of microbubble-based contrast agent for ultrasonography: microscopic studies in rat liver. Invest Radiol 2007; 42: pp. 643-651.
29. Yanagisawa K., Moriyasu F., Miyahara T., et. al.: Phagocytosis of ultrasound contrast agent microbubbles by Kupffer cells. Ultrasound Med Biol 2007; 33: pp. 318-325.