Rationale and Objectives
In this preclinical proof-of-principle study, the necrosis avid agent hypericin was investigated as a potential early indicator for therapeutic response after ethanol-mediated chemical ablation in murine liver tumors.
Materials and Methods
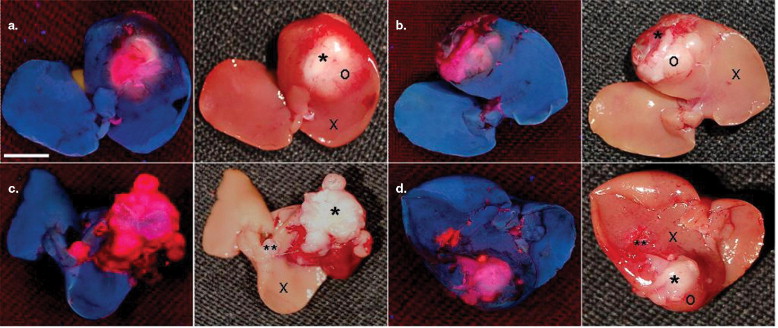
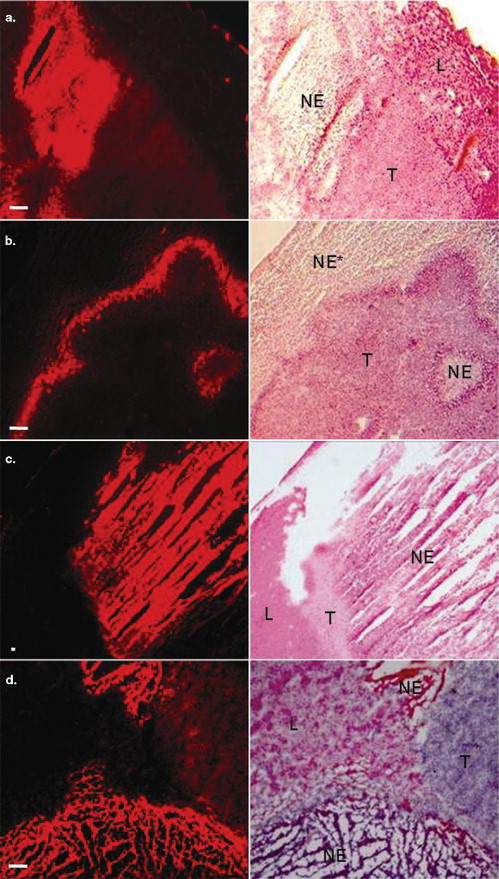
Seven mice bearing intrahepatic radiation-induced fibrosarcoma-1 tumors were intravenously injected with hypericin 1 hour before ( n = 3) or 24 hours after ( n = 4) intratumoral ethanol injection. Mice were euthanized 24 hours after hypericin injection and, taking advantage of the fluorescent property of the compound, the excised livers were investigated qualitatively and quantitatively by means of fluoromacroscopic and fluoromicroscopic examinations, colocalized with conventional histomorphology.
Results
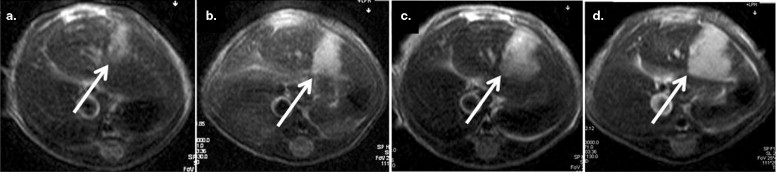
Significant differences in hypericin fluorescence were found in necrosis, viable tumor and normal liver tissue in decreasing order ( P < .05) (ie, in necrosis, mean fluorescence densities were about 4.5 times higher than in viable tumor and approximately 14 times higher than in normal liver). When hypericin was injected 1 hour before, maximal blood concentrations were achieved at the time of ethanol treatment, so that on ablation an outstanding extravasation took place in the entire necrotic area in comparison with accumulation of hypericin only at the peripheral zone of necrosis when it was injected 24 hours after ablation.
Conclusions
Hypericin specifically enhanced the imaging contrast between necrotic and viable tissues and nonspecifically distinguished viable tumor from normal liver. Injection of hypericin shortly before ablation is more favorable than after ablation, because it circumvents difficulties with no-entry zones for hypericin and requires shorter intervals between ethanol ablation and imaging.
Percutaneous ethanol injection (PEI) is a minimally invasive treatment, which has been clinically applied for a couple of decades as an effective alternative to surgery for patients with small hepatocellular carcinoma. Instead of excising the entire tumor from the patient as in standard surgery, PEI serves as a therapy of chemical ablation to instantaneously kill the tumor in situ by intratumoral injection of absolute ethanol. Complete necrosis of lesions smaller than 3 cm in diameter has been achieved ( ). However, as a result of difficulties in obtaining homogeneous intratumoral alcohol distribution, incomplete therapeutic effect may occur because of residual viable neoplastic tissue in tumors larger than 3 cm ( ). This may lead to tumor recurrence particularly along the periphery of the treated area ( ).
A troublesome flaw related to PEI treatment is the difficulty to confirm complete necrosis early after treatment. Early detection of a residual or locally recurrent tumor has a major effect on the prognosis and adjuvant treatment, whereas late diagnosis is associated with peripheral regrowth and remote metastasis making retreatment difficult ( ). Because biopsies and serum tumor markers are only of limited usefulness for assessing the response to PEI, evaluation of the therapeutic effect of PEI is based mainly on findings at imaging studies ( ). However, the visual effects created with all currently applied in vivo imaging techniques (eg, magnetic resonance imaging [MRI], computed tomography, ultrasound [US] imaging) are nonspecific, indirect, and inaccurate. On contrast enhanced MRI, a thin and regular enhancing halo, from an inflammatory reaction, is often observed at the periphery of the hypointense necrotic lesion ( ). Because this strongly enhanced halo may mimic residual tumor, interpretation of enhancing areas at the periphery of the treated lesion can be difficult. Only at later follow-up imaging studies, a few months after treatment, the halo disappears ( ). The use of color Doppler US and contrast-enhanced gray-scale US imaging is only recommended to monitor the response of the tumor during the course of PEI treatment from a substantial risk for false-negative results in postprocedural imaging ( ). Therefore, with the current diagnostic techniques, it is difficult to make a clear-cut distinction between the ablated dead tissues and unablated viable tissues early after PEI treatment.
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Materials and methods
Animals and Tumor System
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Intratumoral Ethanol Injection
Get Radiology Tree app to read full this article<
Hypericin Preparation and Administration
Get Radiology Tree app to read full this article<
Fluoromacroscopic and Fluoromicroscopic Examinations
Get Radiology Tree app to read full this article<
Imaging Quantitative Analyses
Get Radiology Tree app to read full this article<
Results
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Table 1
Ratios of Mean Fluorescence Densities in Different Regions of Fluoromicroscopic Images Taken From 5-μm Tumor Sections
Δ T NE Tissue Viable T NE Tissue Normal L Viable T Normal L 24 h 5.53 ± 2.29 (ns) 15.20 ± 8.89 (ns) 3.84 ± 1.24 (ns) 1 h 3.54 ± 1.12 13.13 ± 5.82 3.05 ± 1.86
Tumors were excised from mice that received 10 mg/kg hypericin either 24 h after (Δ T = 24 h), or 1 h before ethanol treatment (Δ T = 1 h). Statistical analysis, comparing (Δ T = 24 h) with (Δ T = 1 h) was performed using Prism 4.00, GraphPad Software (San Diego, CA). A two-way analysis of variance with Bonferroni test was performed and P values of P < .05 were considered statistically significant. NE: necrotic; T: tumor; L: liver; ns: non-significant.
Get Radiology Tree app to read full this article<
Discussion
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
References
1. Steele G., Ravikumar T.: Resection of hepatic metastases from colorectal cancer. Ann Surg 1989; 210: pp. 127-138.
2. Livraghi T., Festi D., Monti F., et. al.: US-guided percutaneous alcohol injection of small hepatic and abdominal tumors. Radiology 1986; 161: pp. 309-312.
3. Shiina S., Yasuda H., Muto H., et. al.: Percutaneous ethanol injection in the treatment of liver neoplasms. Am J Roentgenol 1987; 149: pp. 949-952.
4. Bartolozzi C., Lencioni R., Caramella D., et. al.: Hepatocellular carcinoma: CT and MR features after transcatheter arterial embolization and percutaneous ethanol injection. Radiology 1994; 191: pp. 123-128.
5. Lin X., Lin L.: Local injection therapy for hepatocellular carcinoma. Hepatobil Pancreat Dis Int 2006; 5: pp. 16-21.
6. Vogl T., Eichler K., Zangos S., et. al.: Hepatocellular carcinoma: role of imaging diagnostics in detection, intervention and follow-up. Rofo 2002; 174: pp. 1358-1368.
7. Bartolozzi C., Lencioni R.: Ethanol injection for the treatment of hepatic tumours. Eur Radiol 1996; 6: pp. 682-696.
8. Nagel H., Bernardino M.: Contrast-enhanced MR imaging of hepatic lesions treated with percutaneous ethanol ablation therapy. Radiology 1993; 189: pp. 265-270.
9. Cioni D., Lencioni R., Bartolozzi C.: Percutaneous ablation of liver malignancies: imaging evaluation of treatment response. Eur J Ultrasound 2001; 13: pp. 73-93.
10. Ni Y., Bormans G., Chen F., et. al.: Necrosis avid contrast agents: functional similarity versus structural diversity. Invest Radiol 2005; 40: pp. 526-535.
11. Ni Y., Chen F., Mulier S., et. al.: Magnetic resonance imaging after radiofrequency ablation in a rodent model of liver tumor: tissue characterization using a novel necrosis avid contrast agent. Eur Radiol 2006; 16: pp. 1031-1040.
12. Giese A.: Hypericism. Photochem Photobiol Rev 1980; 5: pp. 229-255.
13. Kartnig T., Gobel I., Heydel B.: Production of hypericin, pseudohypericin and flavonoids in cell cultures of various Hypericum species and their chemotypes. Planta Med 1996; 62: pp. 51-53.
14. D’Hallewin M., De Witte P., Waelkens E., et. al.: Fluorescence detection of flat bladder carcinoma in situ after intravesical instillation of hypericin. J Urol 2000; 164: pp. 349-351.
15. Kamuhabwa A., Agostinis P., Ahmed B., et. al.: Hypericin as a potential phototherapeutic agent in superficial transitional cell carcinoma of the bladder. Photochem Photobiol Sci 2004; 3: pp. 772-780.
16. Ni Y., Huyghe D., Verbeke K., et. al.: First preclinical evaluation of mono-[123I]iodohypericin as a necrosis-avid tracer agent. Eur J Nucl Med Mol Imaging 2006; 33: pp. 595-601.
17. Twentyman P., Brown J., Gray J., et. al.: A new mouse tumor model system (RIF-1) for comparison of end-point studies. J Natl Cancer Inst 1980; 64: pp. 595-604.
18. Chen F., Sun X., De Keyzer F., et. al.: Liver tumor model with implanted rhabdomyosarcoma in rats: MR imaging, microangiography, and histopathologic analysis. Radiology 2006; 239: pp. 554-562.
19. Falk H., Meyer J., Oberreiter M.: A convenient semisynthetic route to hypericin. Monatsh Chem 1993; 124: pp. 339-341.
20. Ni Y., Marchal G.: Enhanced magnetic resonance imaging for tissue characterization of liver abnormalities with hepatobiliary contrast agents: an overview of preclinical animal experiments. Topics in Magn Reson Imaging 1998; 9: pp. 183-195.
21. Chen B., Xu Y., Roskams T., Delaey E., et. al.: Efficacy of antitumoral photodynamic therapy with hypericin: relationship between biodistribution and photodynamic effects in the RIF-1 mouse tumor model. Int J Cancer 2001; 93: pp. 275-282.
22. Guan Y., Sun L., Zhou X., et. al.: Hepatocellular carcinoma treated with interventional procedures: CT and MRI follow-up. World J Gastroenterol 2004; 10: pp. 3543-3548.