Rationale and Objectives
To investigate immobilization-induced ventilation defects when performing hyperpolarized 3 He (H 3 He) magnetic resonance imaging (MRI) of the lung.
Methods and Materials
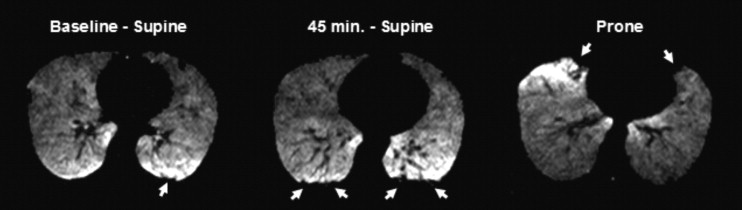
Twelve healthy subjects underwent MRI of the lungs after inhalation of H 3 He gas at three time points: 1) immediately after having been positioned supine on the MRI scanner table, 2) at 45 minutes while remaining supine, 3) and immediately thereafter after having turned prone. All image sets were reviewed in random order by three independent, blinded readers who recorded number, location, and size of H 3 He ventilation defects. Scores were averaged for each time point and comparisons were made to determine change in number, location, and size of ventilation defects with time and positioning of the subject in the scanner.
Results
At baseline supine, there were small numbers of defects in the dependent (posterior) and nondependent (anterior) portions of the lung ( P = .625). At 45 minutes, there was a significant increase in the mean number of ventilation defects/slice (VDS) for the dependent ( P = .005) and a decrease for the nondependent lung portions ( P = .021). After subjects turned prone, mean VDS for posterior defects decreased significantly ( P = .011), whereas those for anterior defects increased ( P = .010). Most defects were less than 3 cm in diameter.
Conclusion
It was found that immobilization of the subject for an extended period led to increased number of H 3 He ventilation defects in the dependent portions of the lung. Therefore, after a subject is positioned in the scanner, H 3 He MR imaging should be performed quickly to avoid the occurrence of the immobilization-induced ventilation defects and possible overestimation of disease.
Hyperpolarized helium-3 (H 3 He) is a gaseous magnetic resonance imaging (MRI) contrast agent that, when inhaled, can be used to evaluate the airspaces of the lung. With this technique, high spatial resolution MRIs of lung ventilation can be obtained, as has been shown in numerous studies involving normal volunteers and patients with a variety of diseases ( ). Although there is generally homogeneous distribution of the MRI signal throughout the lung in healthy subjects after inhalation of the gas, small peripheral ventilation defects in the dependent areas of the lungs can been seen and are believed to be caused by collapse of small portions of the lung ( ). The purpose of this study was to investigate the effect of patient positioning and time on the frequency, size, and location of these defects to better understand their impact and significance in the diagnosis of pulmonary disease when using H 3 He MRI. Our findings were compared with earlier work done in posture-dependence of lung function, in animal studies and patients, using a variety of other modalities ( ).
Materials and methods
Subjects
The study group consisted of 12 healthy volunteers who had never smoked (6 men and 6 women; age range, 22–43 years; median age, 34 years) and had no history of lung disease. None had undergone H 3 He MRI of the lungs previously. All volunteers had to meet the following criteria: normal physical examination, normal spirometric results (predicted forced expiratory volume in one second [FEV 1 ] of 80% or greater); ratio of FEV 1 to forced vital capacity [FEV 1 /FVC] >0.70), normal chest radiography, >95% oxygen saturation at pulse oximetry, and no known allergies. All studies were performed with approval from the Food and Drug Administration for using H 3 He as an Investigational New Drug (IND 57,866) and under a protocol approved by our institution’s investigational review board. All volunteers provided informed written consent before their studies.
3 He Polarization
Get Radiology Tree app to read full this article<
MR Imaging
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Image Analysis
Get Radiology Tree app to read full this article<
Statistical Analysis
Get Radiology Tree app to read full this article<
Results
Get Radiology Tree app to read full this article<
Table 1
Presentation of the Mean Number (± Standard Deviation) of Hyperpolarized 3 He Magnetic Resonance Imaging Ventilation Defects Per Slice for the Group of 12 Subjects Imaged at Three Time Points: Baseline Supine, 45-Minute Supine, and Immediately after Turning Prone
Baseline_P_ ⁎ 45 Minutes_P_ ⁎ Prone_P_ ⁎ Entire lung 0.87 ± 0.89 1.43 ± 0.96 1.00 ± 0.67 Anterior 0.40 ± 0.036 .625 0.24 ± 0.029 .007 0.59 ± 0.076 .058 Posterior 0.47 ± 0.072 1.19 ± 0.161 0.41 ± 0.048 Defects <3 cm 0.69 ± 0.064 .012 1.28 ± 0.154 .034 0.92 ± 0.053 .001 Defects >3 cm 0.17 ± 0.015 0.16 ± 0.016 0.08 ± 0.008 Right Lung 0.43 ± 0.044 .893 0.66 ± 0.112 .428 0.56 ± 0.076 .227 Left Lung 0.44 ± 0.067 0.78 ± 0.145 0.44 ± 0.049 Superior 0.37 ± 0.037 .307 0.66 ± 0.123 .396 0.48 ± 0.062 .500 Inferior 0.50 ± 0.071 0.77 ± 0.136 0.53 ± 0.067
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Discussion
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Conclusion
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Acknowledgments
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
References
1. de Lange E.E., Mugler J.P., Brookeman J.R., et. al.: Lung air spaces: MR imaging evaluation with hyperpolarized 3 He gas. Radiology 1999; 210: pp. 851-857.
2. MacFall J.R., Charles H.C., Black R.D., et. al.: Human lung air spaces: potential for MR imaging with hyperpolarized He-3. Radiology 1996; 200: pp. 553-558.
3. Kauczor H.U., Hofmann D., Kreitner K.F., et. al.: Normal and abnormal pulmonary ventilation: visualization at hyperpolarized He-3 MR imaging. Radiology 1996; 201: pp. 564-568.
4. Donnelly L.F., MacFall J.R., McAdams H.P., et. al.: Cystic fibrosis: combined hyperpolarized 3 he enhanced and conventional proton MR imaging in the lung-preliminary observations. Radiology 1999; 212: pp. 885-889.
5. Altes T.A., Powers P.L., Knight-Scott J., et. al.: Hyperpolarized 3 He MR lung ventilation imaging in asthmatics: preliminary findings. JMRI 2001; 13: pp. 378-384.
6. van Beek E.J., Wild J.M., Kauczor H.U., et. al.: Functional MRI of the lung using hyperpolarized 3-helium gas. J Magn Reson Imaging 2004; 20: pp. 540-554.
7. de Lange E.E., Altes T.A., Patrie J.T., et. al.: Evaluation of asthma with hyperpolarized helium-3 magnetic resonance imaging: correlation with clinical severity and spirometry. Chest 2006; 130: pp. 1055-1062.
8. Nieto M.J., Barba G.P., Mangado N.G., et. al.: Similar ventilation distribution in normal subjects prone and supine during tidal breathing. J Appl Physiol 2002; 92: pp. 622-626.
9. Hedenstierna G.: Atelectasis and gas exchange during anesthesia. Electromedica 2003; 71: pp. 70-73.
10. Warner D.O.: Preventing postoperative pulmonary complications: the role of the anesthesiologist. Anesthesiology 2000; 92: pp. 1467-1472.
11. West J.: Respiratory physiology—the essentials.2004.Lippincott Williams & WilkinsBaltimore, MD:pp. 104.
12. Morimoto S., Takeuchi N., Imanaka H., et. al.: Gravity-dependent atelectasis radiologic, physiologic and pathologic correlation in rabbits on high-frequency oscillation ventilation. Invest Radiol 1989; 24: pp. 522-530.
13. Raoof S., Chowdhrey N., Raoof S., et. al.: Clinical investigations in clinical care. Chest 1999; 115: pp. 1658-1665.
14. Tomiyama N., Takeuchi N., Imanaka H., et. al.: Mechanism of gravity-dependent atelectasis, analysis by nonradioactive xenon-enhanced dynamic computed tomography. Invest Radiol 1993; 28: pp. 633-638.
15. Tusman G., Bohm S., Tempra A., et. al.: Effects of recruitment maneuver on atelectasis in anesthetized children. Anesthesiology 2003; 98: pp. 14-22.
16. Fichele S., Woodhouse N., Swift A., et. al.: MRI of helium-3 gas in healthy lungs: posture related variations of alveolar size. JMRI 2004; 20: pp. 331-335.
17. West J.: Distribution of mechanical stress in the lung, a possible factor in localization of pulmonary disease. Lancet 1971; 1: pp. 839-841.
18. West J., Matthews F.: Stresses, strains and surface pressure in the lung caused by its weight. J Appl Physiol 1972; 32: pp. 332-345.
19. Hoffman E.A.: Effect of body orientation on regional lung expansion: a computed tomographic approach. J Appl Physiol 1985; 59: pp. 468-480.
20. Margulies S., Rodarte J.: Shape of the chest wall in the prone and supine anesthetized dog. J Appl Physiol 1990; 68: pp. 1970-1978.
21. Herold C.J., Kuhlman J.E., Zerhouni E.A.: Pulmonary atelectasis: signal patterns with MR imaging. Radiology 1991; 178: pp. 715-720.
22. Marcucci C., Nyhan D., Simon B.: Distribution of pulmonary ventilation using Xe-enhanced computed tomography in prone and supine dogs. J Appl Physiol 2001; 90: pp. 421-430.
23. Wright F.W.: Atelectasis or collapse?. British Journal of Radiology 2001; 74: pp. 874-875.
24. Crawford A., Cotton D., Paiva M., Engel L.: Effect of airway closure on ventilation distribution. J Appl Physiol 1989; 66: pp. 2511-2515.
25. Mullan B.F., Galvin J.R., Zabner J., Hoffman E.A.: Evaluation of in Vivo Total and Regional Air Content and Distribution in Primate Lungs with High-Resolution CT. Academic Radiology 1997; 4: pp. 674-679.