Rationale and Objectives
The aim of this study is to compare two subjective methods for the identification of changes suggestive of early interstitial lung disease (ILD) on chest computed tomographic (CT) scans.
Materials and Methods
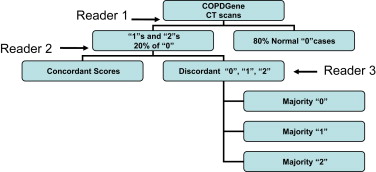
The CT scans of the first 100 subjects enrolled in the COPDGene Study from a single institution were examined using a sequential reader and a group consensus interpretation scheme. CT scans were evaluated for the presence of parenchymal changes consistent with ILD using the following scoring system: 0 = normal, 1 = equivocal for the presence of ILD, 2 = highly suspicious for ILD, and 3 = classic ILD changes. A statistical comparison of patients with early ILD to normal subjects was performed.
Results
There was a high degree of agreement between methods (κ = 0.84; 95% confidence interval, 0.73–0.94; P < .0001 for the sequential and consensus methods). The sequential reading method had both high positive (1.0) and negative (0.97) predictive values for a consensus read despite a 58% reduction in the number of chest CT evaluations. Regardless of interpretation method, the prevalence of chest CT changes consistent with early ILD in this subset of smokers from COPDGene varied between 5% and 10%. Subjects with early ILD tended to have greater tobacco smoke exposure than subjects without early ILD ( P = .053).
Conclusions
A sequential CT interpretation scheme is an efficient method for the visual interpretation of CT data. Further investigation is required to independently confirm our findings and further characterize early ILD in smokers.
Idiopathic pulmonary fibrosis (IPF) is the most common idiopathic interstitial lung disease (ILD), with an estimated 3-year survival rate of 50% . IPF is typically diagnosed 3 to 4 years after the development of symptoms, by which time most patients have advanced pulmonary fibrosis that does not respond to therapeutic intervention. Some authors have speculated that the poor response to medical therapy is due to advanced organ remodeling, although it is unclear, if initation of therapy at the earliest stages of disease would alter its clinical course .
Our group has previously demonstrated both chest computed tomographic (CT) and pathologic evidence of early ILD in asymptomatic members in 18 kindreds affected with familial IPF . These findings suggest that screening high risk populations may, in some cases, detect early subclinical stages of ILD. Although early disease detection may lead to a better understanding of the natural history of IPF, strategies for detection and screening in high-risk populations have not been formally investigated. To address this issue, we compared an efficient sequential reading method to a consensus reading method for the identification of early ILD in a population of smokers (both with and without chronic obstructive pulmonary disease [COPD]) enrolled in the COPDGene Study. We report a high degree of correlation between methods and present the comparison of the clinical characteristics of subjects identified as having early ILD in the COPDGene Study.
Materials and methods
COPDGene
Get Radiology Tree app to read full this article<
CT Scans
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
CT Scoring
Get Radiology Tree app to read full this article<
Sequential Reading Method
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Consensus Reading Method
Get Radiology Tree app to read full this article<
Statistical Analysis
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Results
Cohort Demographics
Get Radiology Tree app to read full this article<
Table 1
Demographic and Functional Data of the Study Cohort ( n = 100)
Characteristic Value Men 44 Age (y) 61.5 (56.6–67.1) Age started smoking (y) 16 (14.5–18.5) Current smoker 47 Tobacco history (pack-years) 37.6 (24.2–53.9) FEV 1 (% predicted) 87 (73–100) FVC (% predicted) 94.5 (83–103.5) FEV 1 /FVC 0.73 (0.63–0.81) Normal spirometric results 61 GOLD stage 1 11 2 20 3 8 4 0 6-min walking distance (ft) 1695 (1400–1860)
FEV 1 , forced expiratory volume in 1 second; FVC, forced vital capacity; GOLD, Global Initiative for Obstructive Lung Disease.
Data are expressed as numbers or as median (interquartile range).
Get Radiology Tree app to read full this article<
Sequential Reading Method
Get Radiology Tree app to read full this article<
Table 2
Comparison of the CT Scan Evaluation Methods
Score Analysis 0 1 2 Number of Readings Sequential 69 23 8 166 Consensus 64 26 10 400
CT, computed tomographic.
In sequential analysis, each scan was reviewed in series, and in consensus analysis, each score was agreed on by three simultaneous readers. The number of readings was calculated as the total number of CT interpretations. For the correlation between these two methods, κ = 0.84 (95% confidence interval, 0.73–0.94; P < .0001).
Get Radiology Tree app to read full this article<
Consensus Reading Method
Get Radiology Tree app to read full this article<
Table 3
Computed Tomographic Findings of the 10 Patients Assigned Scores of 2 by Consensus Review
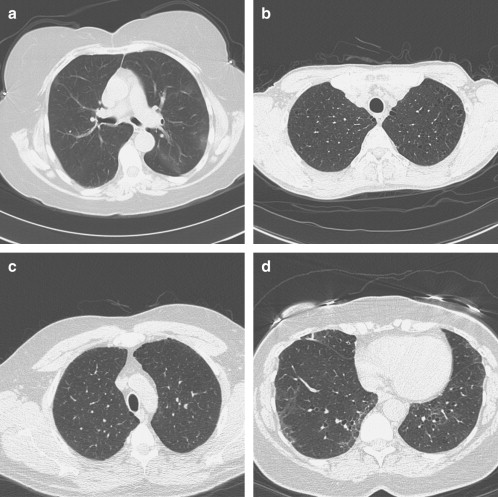
Patient Description 1 ∗ Mild basal asymmetric reticular abnormality with septal thickening 2 Patchy upper lobe ground-glass opacities 3 ∗ Upper lobe irregular cysts 4 Basal linear scarring or dependent ground glass 5 Mild basal peripheral ground glass 6 Basal bronchiectasis and centrilobular nodules, right basal ground glass 7 Upper lobe centrilobular nodules with mild ground glass 8 Mild basal asymmetric reticular and ground glass 9 Patchy bilateral basal ground-glass and reticular abnormality and traction bronchiectasis 10 Mild asymmetric bibasilar ground glass with traction bronchiectasis
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Comparison of Reading Methods
Get Radiology Tree app to read full this article<
Clinical Characteristics of Early ILD by Consensus Reading
Get Radiology Tree app to read full this article<
Table 4
Clinical Characteristics of COPDGene Subjects Stratified by Early ILD Status
Characteristic Score 0 ( n = 65) Score 2 ( n = 10)P ∗ Age (y) 61 (56–65) 64 (60–69) .40 Age started smoking (y) 17 (15–18) 17 (14–18) .80 Women 36 (55%) 5 (50%) 1.00 White 57 (88%) 9 (90%) 1.00 COPD diagnosis † 16 (25%) 3 (30%) .70 Current smokers 27 (42%) 7 (70%) .20 Tobacco history (pack-years) 33 (23–48) 43 (33–90) .053 FEV 1 (% predicted) ‡ 93 (77–108) 82 (79–99) .20 FVC (% predicted) ‡ 101 (90–109) 89 (86–102) .20 FEV 1 /FVC 0.73 (0.61–0.82) 0.69 (0.66–0.76) .70 6-min walking distance (ft) 1700 (1400–1861) 1739 (1560–1780) .70
COPD, chronic obstructive pulmonary disease; FEV 1 , forced expiratory volume in 1 second; FVC, forced vital capacity; GOLD, Global Initiative for Obstructive Lung Disease; ILD, interstitial lung disease.
A comparison of the differences in the clinical characteristics of subjects with computed tomographic findings suggestive of early ILD subjects and normal subjects from the COPDGene study identified by the consensus chest computed tomographic reading method is presented. Data are expressed as median (interquartile range) or as numbers (percentage).
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Discussion
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
References
1. King T.E.J., Tooze J.A., Schwarz M.I., et. al.: Predicting survival in idiopathic pulmonary fibrosis. scoring system and survival model. Am J Respir Crit Care Med 2001; 164: pp. 1171-1181.
2. Raghu G., Weycker D., Edelsberg J., et. al.: Incidence and prevalence of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2006; 174: pp. 810-816.
3. Gross T.J., Hunninghake G.W.: Idiopathic pulmonary fibrosis. N Engl J Med 2001; 345: pp. 517-525.
4. Demedts M., Behr J., Buhl R., et. al.: High-dose acetylcysteine in idiopathic pulmonary fibrosis. N Engl J Med 2005; 353: pp. 2229-2242.
5. King T.E., Behr J., Brown K.K., et. al.: BUILD-1: a randomized placebo-controlled trial of bosentan in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2008; 177: pp. 75-81.
6. Rosas I.O., Ren P., Avila N.A., et. al.: Early interstitial lung disease in familial pulmonary fibrosis. Am J Respir Crit Care Med 2007; 176: pp. 698-705.
7. Lynch D.A., Sahin H., Garg K.: Prevalence of infiltrative lung disease identified on ct in participants in the national lung screening trial. Abstract RSNA 2008; SSQ04–04:609
8. Fisher R.A.: On the interpretation of X2 from contingency tables, and the calculation of P. J R Stat Soc 1922; 85: pp. 87094.
9. Wilcoxon F.: Individual comparisons by ranking methods. Biometrics 1945; 1: pp. 80-83.
10. Rabe K.F., Hurd S., Anzueto A., et. al.: Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2007; 176: pp. 532-555.
11. Hankinson J.L., Odencrantz J.R., Fedan K.B.: Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med 1999; 159: pp. 179-187.
12. Attili A.K., Kazerooni E.A., Gross B.H., et. al.: Smoking-related interstitial lung disease: radiologic-clinical-pathologic correlation. Radiographics 2008; 28: pp. 1383-1396.
13. Gochuico B.R., Avila N.A., Chow C.K., et. al.: Progressive preclinical interstitial lung disease in rheumatoid arthritis. Arch Intern Med 2008; 168: pp. 159-166.
14. Caminati A., Harari S.: Smoking-related interstitial pneumonias and pulmonary Langerhans cell histiocytosis. Proc Am Thorac Soc 2006; 3: pp. 299-306.
15. Baumgartner K.B., Samet J.M., Stidley C.A., et. al.: Cigarette smoking: a risk factor for idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 1997; 155: pp. 242-248.
16. Flaherty K.R., Hunninghake G.G.: Smoking: an injury with many lung manifestations. Am J Respir Crit Care Med 2005; 172: pp. 1070-1071.
17. Selman M., King T.E., Pardo A.: Idiopathic pulmonary fibrosis: prevailing and evolving hypotheses about its pathogenesis and implications for therapy. Ann Intern Med 2001; 134: pp. 136-151.
18. Baran C.P., Opalek J.M., McMaken S., et. al.: Important roles for macrophage colony-stimulating factor, CC chemokine ligand 2, and mononuclear phagocytes in the pathogenesis of pulmonary fibrosis. Am J Respir Crit Care Med 2007; 176: pp. 78-89.
19. Nogee L.M., Dunbar A.E., Wert S.E., et. al.: A mutation in the surfactant protein C gene associated with familial interstitial lung disease. N Engl J Med 2001; 344: pp. 573-579.
20. Hodgson U., Laitinen T., Tukiainen P.: Nationwide prevalence of sporadic and familial idiopathic pulmonary fibrosis: evidence of founder effect among multiplex families in Finland. Thorax 2002; 57: pp. 338-342.
21. Steele M.P., Speer M.C., Loyd J.E., et. al.: Clinical and pathologic features of familial interstitial pneumonia. Am J Respir Crit Care Med 2005; 172: pp. 1146-1152.
22. Marshall R.P., Puddicombe A., Cookson W.O., et. al.: Adult familial cryptogenic fibrosing alveolitis in the United Kingdom. Thorax 2000; 55: pp. 143-146.
23. Bitterman P.B., Rennard S.I., Keogh B.A., et. al.: Familial idiopathic pulmonary fibrosis. Evidence of lung inflammation in unaffected family members. N Engl J Med 1986; 314: pp. 1343-1347.
24. Tsakiri K.D., Cronkhite J.T., Kuan P.J., et. al.: Adult-onset pulmonary fibrosis caused by mutations in telomerase. Proc Natl Acad Sci U S A 2007; 104: pp. 7552-7557.
25. Armanios M.Y., Chen J.J.L., Cogan J.D., et. al.: Telomerase mutations in families with idiopathic pulmonary fibrosis. N Engl J Med 2007; 356: pp. 1317-1326.