Rationale and Objectives
Metastatic involvement of brain is rare in neuroblastoma (NB). We retrospectively evaluated conventional and advanced imaging and clinical findings of seven patients with secondary intra-axial brain NB metastases.
Materials and Methods
Magnetic resonance imaging and computed tomography examinations of patients with metastatic brain NB were reviewed. Recent iodine-123 metaiodobenzylguanidine ( 123 I-MIBG) scans were also reviewed. A medical record review was performed for relevant clinical, laboratory, histopathologic, and genetic data.
Results
Mean age at the time of primary tumor diagnosis was 35 months, and all were considered high-risk NB at diagnosis. Mean time interval between diagnosis and brain involvement was 23.2 months. Extensive prior extra-central nervous system (CNS) disease was present in all patients, but concomitant extra-CNS disease at the time of brain involvement was absent in three (43%) patients. Various forms of disease, including intraparenchymal, intraventricular, and leptomeningeal lesions were detected.
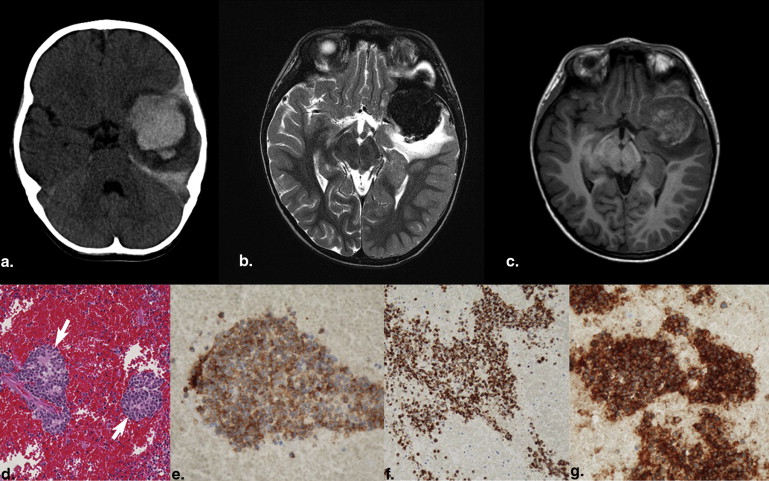
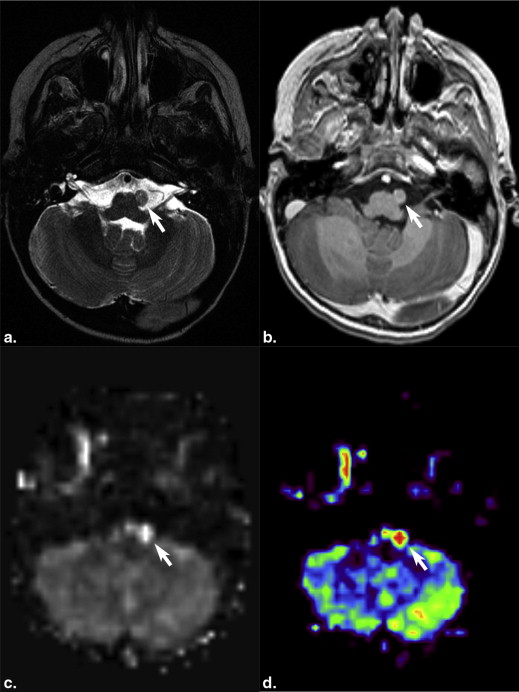
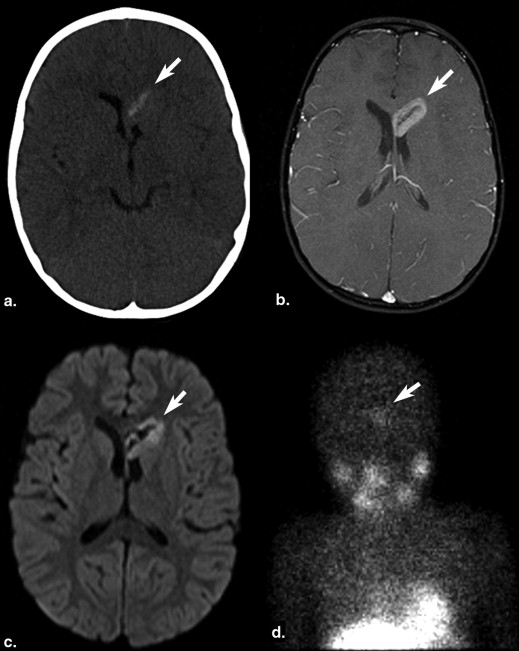
Most intraparenchymal lesions were supratentorial and hemorrhagic; however, hemorrhage was absent in multiple leptomeningeal nodules in one patient. Contrast enhancement of lesions was present on all contrast-enhanced studies. Restricted diffusion of lesions was present in two patients. Arterial spin labeling (ASL) perfusion in two patients also revealed increased cerebral blood flow. Recent 123 I-MIBG scans were available in four patients and showed lesions in two patients with larger metastases but failed to demonstrate lesions in another two patients with smaller lesions.
Conclusions
Brain metastases of NB are often supratentorial and hemorrhagic and demonstrate contrast enhancement. Diffusion-weighted imaging can show restricted diffusion. ASL images may reveal increased perfusion. MIBG scans may not show smaller brain metastases.
Neuroblastoma (NB) is the most common pediatric extracranial neoplasm after leukemia. Metastases are present in up to 70% of patients with NB, frequently involving bone, bone marrow, and liver at the time of diagnosis and confer a poor prognosis. Metastatic involvement of the head and neck is also commonly seen both at presentation and recurrence and manifests most often as osseous metastases involving the calvarium, orbit, or skull base . However, metastasis to the brain (parenchymal, intraventricular, or leptomeningeal) is rare and a serious complication indicating poor prognosis . Recently, the survival of patients with advanced stage NB has improved because of novel treatment protocols that include aggressive chemotherapy, surgery, radiation, autologous stem cell transplantation, and immunotherapy . As life expectancy has increased, brain metastases may be diagnosed more frequently . A single-center study from Memorial Sloan-Kettering Cancer Center revealed a low but increasing incidence of central nervous system (CNS) NB as a complication of stage IV metastatic disease and showed that CNS NB can be the sole site of disease recurrence in 64% of patients, and therefore CNS may represent a sanctuary site for NB . Some recent reports have also suggested that the incidence of CNS recurrence may be increasing , although the increasing incidence of NB metastasis has not been confirmed in all studies .
Get Radiology Tree app to read full this article<
Methods and materials
Patients
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
CT and MR Imaging Protocol
Get Radiology Tree app to read full this article<
Image Analysis
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Histopathology and Genetic Analysis
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Results
Get Radiology Tree app to read full this article<
Table 1
Demographic Data and Outcome of the Study Population
Patient Age at Neuroblastoma Diagnosis (mo) Interval to Brain Metastases (mo) Fate Survival Time After Brain Metastasis (mo) Time to Death (mo) 1 33 11 Dead 1 2 37 12 Alive 1 3 21 17 Alive Not known 4 21 32 Alive 20 5 39 32 Dead 18 6 44 32 Alive 15 7 50 27 Dead 4
Get Radiology Tree app to read full this article<
Imaging Findings
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Table 2
Imaging Findings of Patients with CNS Neuroblastoma
Patient Number of Lesions Location Size (mm) Imaging Modality Enhancement Hemorrhage Diffusion Perfusion 123 I MIBG Scan 1 2 Right temporal and right frontal 41 × 45 × 39 and 21 × 15 × 13 CECT Yes Could not be evaluated N/A N/A 5 d before CT: revealed two lesions. 2 Numerous Left temporal + numerous leptomeningeal brain and spine 46 × 44 × 40 CT and CEMRI Yes Yes Restricted (as measured in largest lesion) ADC = 0.0007902 High CBF max = 251, mean = 242.9 29 d before: revealed no lesion; 1 d after surgery: revealed no lesion, but multiple lesions were present with largest being 8 × 7 × 7 mm 3 1 Right frontotemporal 66 × 62 × 51 CT and CEMRI Yes Yes Distorted N/A Not available 4 1 left frontotemporal 63 × 55 × 45 CT N/A Yes N/A N/A Not available 5 1 Left temporal Second recurrence: 40 × 39 × 40; two foci of residual disease after second recurrence surgery: 12 × 12 × 12 and 10 × 7 × 5 CEMRI Yes Positive in second recurrence, no hemorrhage in residual disease Distorted High CBF max = 98.4 mean = 92.9 Negative 1 d after positive MRI showing two lesions 6 1 Left cerebellar hemisphere 40 × 42 × 30 CT N/A Yes N/A N/A Not available 7 1 Intraventricular, lateral ventricle 33 × 23 × 15 CEMRI and CT Yes Yes Restricted ADC = 0.0007353 N/A 10 d before MRI: revealed one very faint lesion
ADC, apparent diffusion coefficient; CBF, cerebral blood flow; CECT, contrast-enhanced computed tomography; CEMRI, contrast-enhanced magnetic resonance imaging; CNS, central nervous system; CT, computed tomography; 123 I-MIBG, iodine-123 metaiodobenzylguanidine; MRI, magnetic resonance imaging; N/A, not applicable.
Get Radiology Tree app to read full this article<
Clinical and Genetic Features
Get Radiology Tree app to read full this article<
Table 3
Relevant Clinical, Laboratory, and Genetic Findings of Patients with CNS Neuroblastoma
Patient Risk Type Site of Primary Tumor Treatment Before CNS Relapse Treatment After CNS Relapse Prior Extra-CNS Relapse Prior H and N Metastatic Involvement Concomitant Extra-CNS Disease at the Time of CNS Involvement N-myc Gene Amplification Other Genetic Findings Serum LDH 1 High risk Right adrenal Resection of primary + Chemo + therapeutic MIBG + BMT + local radiation None Yes, BM, multiple bone No Yes, multiple bones Yes Loss of 1p, no ALK amplification, loss of 17p and gains of 17q, and deletion of distal 19p 25338 U/L 2 High risk Left adrenal Resection of primary + Chemo + MIBG + BMT + immunotherapy Debulking of largest intracranial lesion + craniospinal irradiation Yes, BM, multiple bone No No Yes Loss of 1p Not known 3 High risk Left retroperitoneal Resection + Chemo + BMT + immunotherapy + local irradiation Not known Yes, BM, multiple bone No Yes, multiple bones Yes Loss of 1p, no ALK amplification, deletion of 9p, gain of most of 17q, and deletion of distal 19p. Not known 4 High risk Left adrenal Resection + Chemo + BMT + immunotherapy + local irradiation Craniospinal radiation + Ommaya radioimmunotherapy Yes, BM, multiple bone, and right orbit Yes No Yes No ALK amplification 6093 U/L 5 High risk Right adrenal Resection + Chemo + local irradiation Surgery + craniospinal irradiation + immunotherapy + investigational drug Yes, BM, multiple bone No No Yes Mutations in the ALK oncogene Not known 6 High risk Left paraspinal Resection + Chemo + MIBG + local irradiation Surgery + craniospinal irradiation + MIBG Yes, BM, multiple bone No Yes, multiple bones No Not known Not known 7 High risk Right adrenal Resection + Chemo + MIBG + stem cell transplant + local radiation + immunotherapy Surgery + Chemo + autologous stem cell infusion + MIBG + focal cranial radiation Yes, BM, multiple bone + lung No Yes, left abdomen No No ALK amplification, loss of 1p, LOH of 3p, relative loss of chromosome 10 Not known
ALK, anaplastic lymphoma kinase; BM, bone marrow; BMT, bone marrow transplantation; Chemo, chemotherapy; CNS, central nervous system; H and N, head and neck; LDH, lactate dehydrogenase; LOH, Loss of heterozygosity; MIBG, metaiodobenzylguanidine scan.
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Discussion
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Conclusion
Get Radiology Tree app to read full this article<
References
1. Chirathivat S., Post M.J.: CT demonstration of dural metastases in neuroblastoma. J Comput Assist Tomogr 1980; 4: pp. 316-319.
2. Zimmerman R.A., Bilaniuk L.T.: CT of primary and secondary craniocerebral neuroblastoma. AJR Am J Roentgenol 1980; 135: pp. 1239-1242.
3. Matthay K., Brisse H., Couanet D., et. al.: Central nervous system metastases in neuroblastoma: radiologic, clinical, and biologic features in 23 patients. Cancer 2003; 98: pp. 155-165.
4. Kramer K., Kushner B., Heller G., et. al.: Neuroblastoma metastatic to the central nervous system. The Memorial Sloan-Kettering Cancer Center experience and a literature review. Cancer 2001; 91: pp. 1510-1519.
5. Zage P.E., Kletzel M., Murray K., et. al.: Outcomes of the POG 9340/9341/9342 trials for children with high-risk neuroblastoma: a report from the Children’s Oncology Group. Pediatr Blood Cancer 2008; 51: pp. 747-753.
6. Blatt J., Fitz C., Mirro J.J.: Recognition of central nervous system metastases in children with metastatic primary extracranial neuroblastoma. Pediatr Hematol Oncol 1997; 14: pp. 233-241.
7. Astigarraga I., Lejarreta R., Navajas A., et. al.: Secondary central nervous system metastases in children with neuroblastoma. Med Pediatr Oncol 1996; 27: pp. 529-533.
8. Wong E.C., Buxton R.B., Frank L.R.: Implementation of quantitative perfusion imaging techniques for functional brain mapping using pulsed arterial spin labeling. NMR Biomed 1997; 10: pp. 237-249.
9. Luh W.M., Wong E.C., Bandettini P.A., et. al.: QUIPSS II with thin-slice TI1 periodic saturation: a method for improving accuracy of quantitative perfusion imaging using pulsed arterial spin labeling. Magn Reson Med 1999; 41: pp. 1246-1254.
10. D’Ambrosio N., Lyo J.K., Young R.J., et. al.: Imaging of metastatic CNS neuroblastoma. AJR Am J Roentgenol 2010; 194: pp. 1223-1229.
11. Palasis S., Egelhoff J.C., Morris J.D., et. al.: CNS relapse of treated stage IV neuroblastoma. Pediatr Radiol 1998; 28: pp. 990-994.
12. Dresler S., Harvey D.G., Levisohn P.M.: Retroperitoneal neuroblastoma widely metastatic to the central nervous system. Ann Neurol 1979; 5: pp. 196-198.
13. Krol G., Horten B.: Neuroblastoma metastatic to the leptomeninges. Clini Bull 1978; 8: pp. 120-122.
14. Codreanu I., Dasanu C.A., Zhuang H.: Neuroblastoma with a solitary intraventricular brain metastasis visualized on I-123 MIBG scan. J Neuroimaging 2012; Epub ahead of print
15. Palasis S., Morris J., Egelhoff J.: Central nervous system relapse following bone marrow transplantation in stage IV neuroblastoma. Pediatr Hematol Oncol 1999; 16: pp. 443-452.
16. Porto L., Kieslich M., Yan B., et. al.: Isolated CNS relapse in neuroblastoma. Neuropediatrics 2005; 36: pp. 112-116.
17. Brodeur G.M., Seeger R.C., Schwab M., et. al.: Amplification of N-myc in untreated human neuroblastomas correlates with advanced disease stage. Science 1984; 224: pp. 1121-1124.
18. Mosse Y.P., Laudenslager M., Khazi D., et. al.: Germline PHOX2B mutation in hereditary neuroblastoma. Am J Hum Genet 2004; 75: pp. 727-730.
19. Mossé Y.P., Laudenslager M., Longo L., et. al.: Identification of ALK as a major familial neuroblastoma predisposition gene. Nature 2008; 455: pp. 930-935.
20. Chen Y., Takita J., Choi Y.L., et. al.: Oncogenic mutations of ALK kinase in neuroblastoma. Nature 2008; 455: pp. 971-974.
21. Stallings R.L., Nair P., Maris J.M., et. al.: High-resolution analysis of chromosomal breakpoints and genomic instability identifies PTPRD as a candidate tumor suppressor gene in neuroblastoma. Cancer Res 2006; 66: pp. 3673-3680.
22. Molenaar J.J., Koster J., Zwijnenburg D.A., et. al.: Sequencing of neuroblastoma identifies chromothripsis and defects in neuritogenesis genes. Nature 2012; 483: pp. 589-593.
23. Watts R.: Combination chemotherapy with ifosfamide and etoposide is effective in the treatment of central nervous system metastasis of childhood neuroblastoma. Cancer 1992; 69: pp. 3012-3014.
24. Kellie S., Hayes F., Bowman L., et. al.: Primary extracranial neuroblastoma with central nervous system metastases characterization by clinicopathologic findings and neuroimaging. Cancer 1991; 68: pp. 1999-2006.