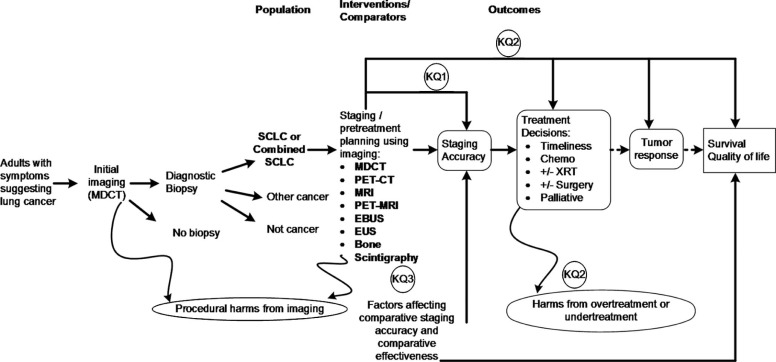
Background
Small cell lung cancer (SCLC) is an aggressive form of lung cancer. Accurate staging is essential to select the optimal treatment plan to maximize survival. No consensus exists on standard imaging modalities for pretreatment staging of SCLC.
Materials and Methods
We conducted a systematic review of the literature on imaging modalities in the pretreatment staging of SCLC. A systematic search of multiple databases identified relevant studies published from 2000 through June 2015. Outcomes of interest included test concordance, staging accuracy (sensitivity and specificity), choice of treatment, timeliness of treatment, and patient outcomes.
Results
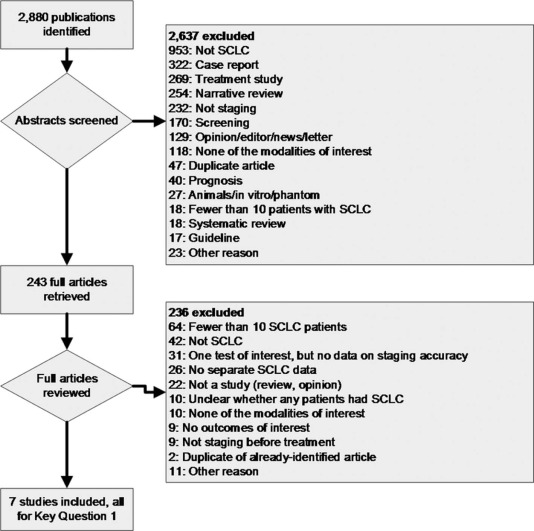
The search identified 2880 citations; 7 studies met inclusion criteria, n = 408 patients. Six of the seven studies were deemed to have moderate risk of bias, and one was deemed to have high risk of bias. One of the studies reported test concordance, three studies reported comparative accuracy of testing strategies, and four studies reported the accuracy of a single imaging modality. Analysis from these studies revealed that fluorodeoxyglucose–positron emission tomography/computed tomography (FDG-PET/CT) is more sensitive than multidetector CT for detecting osseous metastases, more sensitive than bone scintigraphy for detecting osseous metastases, and more sensitive for detecting any distant metastases.
Conclusions
Evidence is sparse on the use of imaging in the pretreatment staging of SCLC. There is a lack of evidence on patient-oriented outcomes and a lack of evidence on whether comparative accuracy or effectiveness is associated with patient factors. We found low-strength evidence suggesting that FDG-PET/CT is more sensitive than CT and bone scintigraphy for detecting osseous metastases.
Introduction
Lung cancer is the leading cause of cancer-related mortality, accounting for about 27% of cancer deaths in the United States in 2014 . Small cell lung cancer (SCLC) is an aggressive form of lung cancer characterized by rapid doubling time, high-growth fraction, and early development of metastatic disease. This histological subset of lung cancer is primarily seen in smokers and accounts for approximately 15% of all lung cancers . Despite advances in diagnosis, treatment, and management of lung cancer, the 5-year survival rate for SCLC remains dismal at about 6% .
Staging involves determining the extent of disease and deciding on the treatment choice. SCLC is often staged using the Veterans Administration Lung Study Group system , which classifies SCLC as either limited stage disease (LD), wherein cancer is confined to one hemithorax, all of which can be encompassed in a safe radiotherapy field, or extensive stage disease (ED), which does not meet the criteria for LD. At diagnosis, the vast majority of patients with SCLC have ED, with overall long-term survival of only 1% . Chemotherapy remains the standard of care for this stage of disease and is associated with improved overall survival and quality of life. LD is treated more aggressively, with a curative intent, with chemoradiation .
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Methods
General
Get Radiology Tree app to read full this article<
Project Scope and Key Questions
Get Radiology Tree app to read full this article<
TABLE 1
Scope of Review
Populations Adult patients with known SCLC or combined SCLC who are undergoing imaging test(s) for staging and who have not yet received treatment. Interventions Imaging using one or more of the following tests:
Comparators
Outcomes
CT, computed tomography; EBUS, endobronchial ultrasound; EUS, endoscopic ultrasound; MDCT, multidetector computed tomography; MRI, magnetic resonance imaging; PET/CT, positron emission tomography/computed tomography; SCLC, small cell lung cancer.
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
TABLE 2
Key Questions
Key Question 1 What are the test concordance and comparative accuracy of imaging tests (MDCT, PET/CT, MRI, PET/MRI, EBUS, EUS, and bone scintigraphy) for the pretreatment staging of SCLC?
Key Question 2 When used for the pretreatment staging of SCLC, what is the comparative effectiveness of imaging tests (MDCT, PET/CT, MRI, PET/MRI, EBUS, EUS, and bone scintigraphy) on later outcomes?
Key Question 3 To what extent are the following factors associated with the comparative accuracy or effectiveness of imaging tests (MDCT, PET/CT, MRI, PET/MRI, EBUS, EUS, and bone scintigraphy) when used for the pretreatment staging of SCLC?
CT, computed tomography; EBUS, endobronchial ultrasound; EUS, endoscopic ultrasound; MDCT, multidetector computed tomography; MRI, magnetic resonance imaging; PET/CT, positron emission tomography/computed tomography; SCLC, small cell lung cancer.
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Literature Search
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Article Screening and Inclusion/Exclusion Criteria
Get Radiology Tree app to read full this article<
TABLE 3
Inclusion Criteria
Publication criteria Published as a full-length article in peer-reviewed journal.
Published on January 1, 2000, or later.
Published in English (this criterion was subsequently removed). Study design Provides data on at least one test of interest.
For comparative accuracy (KQ1), the study tests must have been compared to a common reference standard.
For comparative effectiveness (KQ2), different tests or test strategies must have been given to separate groups of patients.
For comparisons of variants on a modality or studies of patient or tumor characteristics for KQ3, comparisons must have been planned in advance. Patient criteria At least 85% of the patients must have been undergoing staging for SCLC.
At least 85% of the patients must have been aged 18 years or older, or data must have been reported separately for patients aged 18 or older.
Studies for the staging of recurrent SCLC were excluded.
Data on imaging tests performed after any form of treatment were excluded. Test criteria CT must have been performed on a multidetector scanner. Studies that did not explicitly specify whether a single- or multidetector scanner was used were assumed to be MDCT.
PET/CT must have been performed on an integrated PET/CT device. Data criteria At least 10 patients per imaging test or test strategy participated.
Data reported for at least 50% of the patients initially enrolled.
For studies of test accuracy (KQ1), there must be sufficient data to allow calculation of sensitivity and specificity with their confidence intervals.
For studies of test concordance (KQ1), the number of patients with the same and different results must be reported.
For comparisons in KQ2, the choice and/or timeliness of treatment or the outcomes of treatment (such as survival, tumor response, quality of life, or adverse events) must be reported for each study group.
For comparisons in KQ3, comparative accuracy or comparative effectiveness data must be reported for each patient group.
CT, computed tomography; KQ, Key Question; MDCT, multidetector computed tomography; PET/CT, positron emission tomography/computed tomography; SCLC, small cell lung cancer.
Get Radiology Tree app to read full this article<
Data Abstraction
Get Radiology Tree app to read full this article<
Risk-of-bias Appraisal
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Data Synthesis
Get Radiology Tree app to read full this article<
Grading Strength of Evidence
Get Radiology Tree app to read full this article<
Expert Review and Public Review
Get Radiology Tree app to read full this article<
Role of the Funding Source
Get Radiology Tree app to read full this article<
Results
Literature Search
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Key Question 1
Get Radiology Tree app to read full this article<
TABLE 4
Quantity of Evidence
Staging Determination Direct Comparisons between Imaging Modalities Accuracy Data on a Single Imaging Modality (for Indirect Comparisons) Limited or extensive disease Standard staging \* vs FDG-PET/CT; one study; n = 28 Standard staging \* only; one study, n = 25 Presence of metastases to osseous structures (bone or bone marrow) MDCT vs bone scintigraphy; one study; n = 28
MDCT vs FDG-PET/CT; one study, n = 29
Bone scintigraphy vs FDG-PET/CT; two studies, n = 123 Bone scintigraphy only; one study, n = 76 Presence of lymph node involvement None EBUS only; one study, n = 36 Presence of metastases to adrenal glands None MDCT only; one study, n = 120 Presence of metastases to the liver None MDCT only; one study, n = 120 Presence of metastases to the spleen None MDCT only; one study, n = 120 Presence of metastases to the brain None FDG-PET/CT only; one study, n = 21 Presence of any distant metastases Standard staging vs standard staging plus FDG-PET/CT; one study; n = 73 None
CT, computed tomography; FDG-PET/CT, fluorodeoxyglucose positron–emission tomography/computed tomography; EBUS, endobronchial ultrasonography; MDCT, multidetector computed tomography; MRI, magnetic resonance imaging.
Get Radiology Tree app to read full this article<
TABLE 5
Evidence Table
Study Staging Determination Test(s) Test 1 Sensitivity (95% CI) Test 2 Sensitivity (95% CI) Test 1 Specificity (95% CI) Test 2 Specificity (95% CI) Sensitivity Difference a Specificity Difference a Fischer et al LD vs ED Standard staging vs PET/CT 86% (66%–95%) (19/22) 95% (78%–99%) (21/22) 100% (60%–100%) (6/6) 100% (60%–100%) (6/6) Not statistically significant Not statistically significant Shen et al LD vs ED Standard staging only 93% (70%–99%) (14/15) NA 90% (59%–98%) (9/10) NA NA NA Lee et al Metastasis to osseous structures PET/CT vs scintigraphy 100% (88%–100%) (30/30) 37% (22%–55%) (11/30) 100% (94%–100%) (65/65) 92% (83%–97%) (60/65) Statistically significant
Favors PET/CT Not statistically significant Fischer et al Metastasis to osseous structures CT vs scintigraphy b 22% (7%–55%) (2/9) 78% (45%–93%) (7/9) 100% (83%–100%) (19/19) 58% (36%–77%) (11/19) Statistically significant
Favors scintigraphy Statistically significant
Favors CT Fischer et al Metastasis to osseous structures CT vs scintigraphy c 22% (7%–55%) (2/9) 22% (7%–55%) (2/9) 100% (83%–100%) (19/19) 84% (62%–94%) (16/19) Not statistically significant Not statistically significant Fischer et al Metastasis to osseous structures CT vs PET/CT 30% (11%–60%) (3/10) 80% (49%–94%) (8/10) 100% (83%–100%) (19/19) 100% (83%–100%) (19/19) Statistically significant
Favors PET/CT Not statistically significant Fischer et al Metastasis to osseous structures PET/CT vs scintigraphy b 78% (45%–93%) (7/9) 78% (45%–93%) (7/9) 100% (83%–100%) (19/19) 58% (36%–77%) (11/19) Not statistically significant Statistically significant
Favors PET/CT Fischer et al Metastasis to osseous structures PET/CT vs scintigraphy c 78% (45%–93%) (7/9) 22% (7%–55%) (2/9) 100% (83%–100%) (19/19) 84% (62%–94%) (16/19) Statistically significant
Favors PET/CT Not statistically significant Brink et al Metastases to osseous structures Scintigraphy only 61% (41%–78%) (14/23) NA 96% (87%–99%) (51/53) NA NA NA Wada et al Metastasis to mediastinal and hilar lymph nodes EBUS only 96% (82%–99%) (27/28) NA 100% (67%–100%) (8/8) NA NA NA Brink et al Metastasis to lymph node(s) d CT only 70% (56%–80%) (37/53) NA 94% (85%–97%) (61/65) NA NA NA Brink et al Metastasis to adrenal glands CT only 63% (43%–79%) (15/24) NA 96% (90%–98%) (92/96) NA NA NA Brink et al Metastasis to liver CT only 88% (71%–96%) (23/26) NA 98% (92%–99%) (92/94) NA NA NA Brink et al Metastasis to spleen CT only 75% (30%–95%) (3/4) NA 100% (97%–100%) (116/116) NA NA NA Sohn et al e Distant metastasis Standard staging vs PET/CT 46% (29%–65%) (12/26) 92% (76%–98%) (24/26) 100% (86%–99%) (47/47) 96% (92%–100%) (45/47) Statistically significant
Favors PET/CT Not statistically significant Palomar Munoz et al Metastasis to brain PET/CT of the brain 60% (23%–88%) (3/5) NA 100% (80%–100%) (16/16) NA NA NA
CI, confidence interval; CT, computed tomography; EBUS, endobronchial ultrasound; ED, extensive stage disease; LD, limited stage disease; NA, not applicable; NR, not reported; PET/CT, positron emission tomography/computed tomography.
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
TABLE 6
Conclusions on Comparative Effectiveness
Staging Determination Conclusion Evidence Grade Limited or extensive disease No conclusions possible (imprecise results) Presence of metastases to osseous structures PET/CT more sensitive than scintigraphy
PET/CT more sensitive than CT Low
Low Presence of lymph node involvement No conclusions possible (differing patient populations) Presence of metastases to adrenal glands No conclusions possible (insufficient evidence) Presence of metastases to the liver No conclusions possible (insufficient evidence) Presence of metastases to the spleen No conclusions possible (insufficient evidence) Presence of metastases to the brain No conclusions possible (insufficient evidence) Presence of any distant metastases Standard staging plus PET/CT is more sensitive than standard staging alone. Low
CT, computed tomography; PET/CT, positron emission tomography/computed tomography.
Get Radiology Tree app to read full this article<
Key Question 2
Get Radiology Tree app to read full this article<
Key Question 3
Get Radiology Tree app to read full this article<
Discussion
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Acknowledgments
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Appendix
Supplementary material
Get Radiology Tree app to read full this article<
Appendix S1
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Appendix S2
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
References
1. American Cancer Society (ACS) : Cancer facts & figures 2014.2014.American Cancer Society (ACS)Atlanta (GA)
2. Howlader N., Noone A.M., Krapcho M., et. al.: SEER Cancer Statistics Review, 1975–2011. National Cancer Institute; Bethesda, MD http://seer.cancer.gov/csr/1975_2011/ based on November 2013 SEER data submission, posted to the SEER web site, April 2014
3. National Cancer Institute (NCI) : Small cell lung cancer treatment (PDQ) health professional version. National Institutes of Health Web site; Available at http://www.cancer.gov/cancertopics/pdq/treatment/small-cell-lung/healthprofessional/page1 Accessed: June 11, 2014
4. Shepherd F.A., Crowley J., Van Houtte P., et. al.: The International Association for the Study of Lung Cancer lung cancer staging project: proposals regarding the clinical staging of small cell lung cancer in the forthcoming (seventh) edition of the tumor, node, metastasis classification for lung cancer. J Thorac Oncol 2007; 2: pp. 1067-1077.
5. Kalemkerian G.P., Akerley W., Bogner P., et. al.: Small cell lung cancer. J Natl Compr Canc Netw 2013; 11: pp. 78-98.
6. Micke P., Faldum A., Metz T., et. al.: Staging small cell lung cancer: veterans Administration Lung Study Group versus International Association for the Study of Lung Cancer—what limits limited disease?. Lung Cancer 2002; 37: pp. 271-276.
7. Jett J.R., Schild S.E., Kesler K.A., et. al.: Treatment of small cell lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013; 143: pp. e400S-e419S.
8. Glisson B.S., Byers L.A.: Pathobiology and staging of small cell carcinoma of the lung. UptoDate Web site; Available at http://www.uptodate.com/home/index.html Accessed June 12, 2014
9. Hillner B.E., Tosteson A.N., Song Y., et. al.: Growth in the use of PET for six cancer types after coverage by Medicare: additive or replacement?. J Am Coll Radiol 2012; 9: pp. 33-41.
10. Palomar Munoz A., Garcia Vicente A.M., Bellon Guardia M.E., et. al.: Is a selective brain (18)F-FDG PET/CT study profitable in patients with small cell lung cancer?. Rev Esp Med Nucl Imagen Mol 2012; 31: pp. 124-129.
11. Brink I., Schumacher T., Mix M., et. al.: Impact of ([18]F)FDG-PET on the primary staging of small-cell lung cancer. Eur J Nucl Med Mol Imaging 2004; 31: pp. 1614-1620.
12. Lee J.W., Lee S.M., Lee H.S., et. al.: Comparison of diagnostic ability between (99m)Tc-MDP bone scan and 18F-FDG PET/CT for bone metastasis in patients with small cell lung cancer. Ann Nucl Med 2012; 26: pp. 627-633.
13. Sohn B.S., Lee D.H., Kim E.K., et. al.: The role of integrated 18F-FDG PET-CT as a staging tool for limited-stage small cell lung cancer: a retrospective study. Onkologie 2012; 35: pp. 432-438.
14. Fischer B.M., Mortensen J., Langer S.W., et. al.: A prospective study of PET/CT in initial staging of small-cell lung cancer: comparison with CT, bone scintigraphy and bone marrow analysis. Ann Oncol 2007; 18: pp. 338-345.
15. Shen Y.Y., Shiau Y.C., Wang J.J., et. al.: Whole-body 18F-2-deoxyglucose positron emission tomography in primary staging small cell lung cancer. Anticancer Res 2002; 22: pp. 1257-1264.
16. Lu Y.Y., Chen J.H., Liang J.A., et. al.: 18F-FDG PET or PET/CT for detecting extensive disease in small-cell lung cancer: a systematic review and meta-analysis. Nucl Med Commun 2014; 35: pp. 697-703.
17. Ruben J.D., Ball D.L.: The efficacy of PET staging for small-cell lung cancer: a systematic review and cost analysis in the Australian setting. J Thorac Oncol 2012; 7: pp. 1015-1020.
18. Hellwig D., Baum R.P., Kirsch C.: FDG-PET, PET/CT and conventional nuclear medicine procedures in the evaluation of lung cancer: a systematic review. Nuklearmedizin 2009; 48: pp. 59-69.
19. Ung Y.C., Maziak D.E., Vanderveen J.A., et. al.: 18Fluorodeoxyglucose positron emission tomography in the diagnosis and staging of lung cancer: a systematic review. J Natl Cancer Inst 2007; 99: pp. 1753-1767.
20. Facey F., Bradbury I., Laking G., et. al.: Overview of the clinical effectiveness of positron emission tomography imaging in selected cancers. Health Technol Assess 2007; 11: pp. iii-85.
21. Ravenel J.G., Rosenzweig K.E., Kirsch J., et. al.: ACR appropriateness criteria non-invasive clinical staging of bronchogenic carcinoma. J Am Coll Radiol 2014; 11: pp. 849-856.
22. Wada H., Nakajima T., Yasufuku K., et. al.: Lymph node staging by endobronchial ultrasound-guided transbronchial needle aspiration in patients with small cell lung cancer. Ann Thorac Surg 2010; 90: pp. 229-234.
23. AHRQ Publication No. 10(14)-EHC063-EF2014.Agency for Healthcare Research and Quality (AHRQ)Rockville (MD)
24. AHRQ Publication No. 14-EHC010-EF]2014.Agency for Healthcare Research and Quality (AHRQ)Rockville (MD)
25. Treadwell J.R., Mitchell M.D., Tsou A., et. al.: Imaging for the Pretreatment Staging of Small Cell Lung Cancer. Comparative Effectiveness Review No. 174. (Prepared by the ECRI Institute-Penn Medicine Evidence-based Practice Center under Contract No. 290-2012-00011-I.); AHRQ Publication No. 16-EHC015-EF; Rockville, MD; Agency for Healthcare Research and Quality; April 2016 www.effectivehealthcare.ahrq.gov/reports/final.cfm
26. Dwamena B.: MIDAS: stata module for meta-analytical integration of diagnostic test accuracy studies. Statistical Software Components S456880, Boston College Department of Economics. revised 05 Feb; Available at http://ideas.repec.org/c/boc/bocode/s456880.html Accessed August 12, 2015
27. Trikalinos T.A., Hoaglin D.C., Small K.M., et. al.: Evaluating practices and developing tools for comparative effectiveness reviews of diagnostic test accuracy: methods for the joint meta-analysis of multiple tests. Methods research report. (Prepared by the Tufts Evidence-based Practice Center, under Contract No. 290-2007-10055-I.)AHRQ publication no. 12(13)-EHC151-EF2013.Agency for Healthcare Research and Quality (AHRQ)Rockville (MD)