Rationale and Objectives
This study aimed to assess the clinical efficacy of positron emission tomography (PET) or combined PET-computed tomography (CT) with 18 F-fluorodeoxyglucose (FDG) for whole-body cancer screening in patients with suspected paraneoplastic neurological syndromes (PNS). The following main research questions were addressed: What is the percentage of positive findings to be expected in whole-body FDG-PET-CT in adult patients with PNS? How many false positives can be expected as assessed by clinical and histopathological workup? Are there patients who present with a tumor despite initially negative findings?
Materials and Methods
This is a systematic review of the literature and retrospective analysis of FDG-PET-CT and clinical follow-up data from 45 consecutive patients (age: 56.6 ± standard deviation 15.8 years, 14 female, 31 male). Suspicious lesions were identified and correlated with immediate workup and clinical follow-up.
Results
Fourteen studies were included in the review. Eleven malignancies (24.4% of patients) were identified by FDG-PET-CT in this sample. This is a higher percentage of positive findings compared to most previous reports. There was one initially negative finding.
Conclusions
Whole-body FDG-PET-CT is suitable to identify additional malignancies in patients with suspected classical PNS referred to a tertiary medical center. The utility by means of true-positive findings is higher in classical PNS than suggested by studies in less select patient populations.
Introduction
Paraneoplastic neurological syndromes (PNS) are rare, often subacutely manifesting conditions associated with malignant neoplasms. PNS are not directly caused by the tumor itself, metastases, metabolic derangement, or treatment effects. They are hypothesized to be immune-mediated disorders, and a range of associated autoantibodies have been characterized accordingly . Standardized diagnostic criteria for PNS have been introduced and widely adopted. They differentiate between classical and nonclassical PNS . A clinically diagnosed classical PNS with a high concentration of characteristic antineuronal antibodies is associated with a high probability of an underlying malignancy .
Because of the indirect nature of disease mechanisms and a potential tumor-suppressive effect of the autoimmune reaction, these tumors are often small . Therefore, diagnostic techniques to identify potentially underlying neoplasms have to provide sufficient sensitivity. A multimodal workup guided by the probability of tumor location has been recommended as first-line diagnostic approach . However, in recent years, positron emission tomography (PET) with 18 F-fluorodeoxyglucose (FDG) with or without simultaneous contrast-enhanced computed tomography (CT) has been proposed as a whole-body screening method, particularly in patients with PNS and negative findings in conventional cancer screening . A recent meta-analysis on PET and PET-CT in paraneoplastic syndromes in general (including PNS) has come to the conclusion that the overall accuracy is high in this setting . Evidence of diagnostic accuracy of PET-CT and a potential impact on outcomes in patients with suspected PNS is limited by small sample sizes and a distinct heterogeneity of studies featuring various outcome measures, reference standards, and clinical end points reflecting the rarity of these disorders.
Get Radiology Tree app to read full this article<
Materials and Methods
Systematic Review of the Literature
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Retrospective Analysis of Examinations in a Tertiary Hospital
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Results
Systematic Review of the Literature
Get Radiology Tree app to read full this article<
TABLE 1
Overview of Studies Reporting Diagnostic Accuracies of PET or Combined PET-CT in PNS
Reference Patients PNS Entities Design Imaging Modalities Reference Standards Overall Positives Measures of Diagnostic Accuracy \* Rees et al. 2001 n = 43 Suspected PNS Retrospective 18 F-FDG-PET Follow-up, histopathology (partial) 37.2% True positives = 44% of positives † Berner et al. 2003 n = 30 (mean age = 55 y, range: 22–76 y) Suspected paraneoplastic syndrome (neurological and dermatological) Retrospective 18 F-FDG-PET Follow-up, histopathology (partial) 26.7% True positives = 75% of positives, sensitivity = 86%, specificity = 91% Younes-Mhenni et al. 2004 n = 20 (mean age = 62 y, range: 49–78 y) Suspected PNS with positive autoantibodies Prospective 18 F-FDG-PET Follow-up, histopathology (partial) 80.0% True positives = 78% of positives, sensitivity = 83%, specificity = 25% Patel et al. 2008 n = 104 (median age = 56 y, range: 17–84 y) Suspected PNS Retrospective 18 F-FDG-PET Follow-up, histopathology (partial) 23.1% True positives: 42% of positives † , sensitivity = 80%, specificity = 67% Hadjivassiliou et al. 2009 n = 80 Suspected PNS with no predefined criteria to reflect clinical practice Prospective 18 F-FDG-PET Typical clinical entities or antibodies (not: proven malignancy) 23% True positives: 44% of positives ‡ , sensitivity = 75%, specificity = 87% Bannas et al. 2010 n = 46 (median age = 65 y, range: 21–81 y) Classical PNS in 37%, other suspected PNS in the remaining patients Retrospective 18 F-FDG-PET-CT (positive oral contrast, no intravenous contrast) Follow-up, histopathology (partial) 21.7% True positives: 40% of positives § McKeon et al. 2010 n = 56 (median age = 61 y, range: 22–80 y) Suspected PNS Retrospective 18 F-FDG-PET-CT Follow-up, histopathology (partial) 39% True positives = 50% of positives with further targeted evaluations Selva-O’Callaghan et al. 2010 n = 55 (median age = 58 y, range: 46–69 y) Dermatomyositis and polymyositis Prospective 18 F-FDG-PET-CT Follow-up, histopathology (partial) 12.7% True positives: 86% of positives, sensitivity = 66.7%, specificity = 97.8% Matsuhisa et al. 2012 n = 27 (mean age = 65 y) Suspected PNS Retrospective 18 F-FDG-PET-CT (non-enhanced CT) Follow-up, histopathology (partial) 22% True positives: 83% of positives, false-negative findings in the whole sample: 3.7% Vaidyanathan et al. 2012 n = 68 (median age = 58 y, range: 23–82 y) Suspected paraneoplastic syndrome (81% PNS) Retrospective 18 F-FDG-PET-CT (non-enhanced) Follow-up, histopathology (partial) 26% True positives: 44% of positives, sensitivity = 100%, specificity = 82% Schramm et al. 2013 n = 66 (mean age = 60 y, range: 21–87 y) Suspected PNS Retrospective 18 F-FDG-PET-CT (intravenous contrast in 83%) Follow-up, histopathology (partial) 15% True positives: 90% of positives, sensitivity = 100%, specificity = 90% Kristensen et al. 2015 n = 137 (PNS: n = 67, mean age = 61 y, range: 10–63 y) Suspected paraneoplastic syndrome (predominantly neurological) Retrospective 18 F-FDG-PET-CT (non-enhanced) Follow-up, histopathology (partial) 22.6% True positives = 29% of positives, sensitivity = 75%, specificity = 82% Vatankulu et al. 2016 n = 42 (median age = 58 y, range: 14–82 y) Classical PNS in 75%, other suspected PNS in the remaining patients Retrospective 18 F-FDG-PET-CT (non-enhanced) Follow-up, histopathology (partial) 14% True positives = 100% of positives, sensitivity = 86%, specificity = 100% Pena Pardo et al. 2016 n = 73 (mean age = 61 y, SD: 14 y) Classical PNS in 55%, other suspected PNS in the remaining patients Retrospective 18 F-FDG-PET-CT (non-enhanced) Follow-up, histopathology (partial) 9% (+ 25% classified as indeterminate) True positives = 57% of positives
CT, computed tomography; FDG, F-fluorodeoxyglucose; PET, positron emission tomography; PNS, paraneoplastic neurological syndrome; SD, standard deviation.
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Retrospective Analysis of PET-CT Examinations
Get Radiology Tree app to read full this article<
TABLE 2
Patients with Suspicious Findings in Combined PET-CT
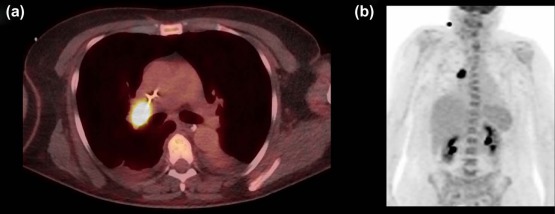
Age, Sex PNS Entity Lesion Location (PET-CT) Tumor Immediate Workup Antineuronal Antibodies 60 y, male Encephalomyelitis Lymph node (mediastinum) SCLC Mediastinoscopy ANNA1 59 y, female Subacute cerebellar degeneration Intramammary nodule, lymph nodes (mediastinal, abdominal) \* Recurrent breast cancer \* Biopsy, extended restaging \* Anti-Yo 65 y, male Subacute cerebellar degeneration lung NSCLC CT-guided biopsy — 53 y, male Limbic encephalitis Lymph node (mediastinum) SCLC † — † ANNA1 68 y, male Subacute sensory neuropathy Lung, lymph nodes (mediastinum) NSCLC ‡ Mediastinoscopy CV2/CRMP5 63 y, female Subacute cerebellar degeneration Retroperitoneal mass Gastric adenocarcinoma Endosonography-guided biopsy — 79 y, male Subacute sensory neuropathy Pancreas, lung Pancreatic adenocarcinoma (ductal) with peritoneal spread Endosonography-guided biopsy (not tested) 60 y, female Limbic encephalitis Lung, lymph nodes (mediastinum, pulmonary hilus) SCLC Bronchoalveolar lavage GABA B -receptor 58 y, male Encephalomyelitis Bone (multifocal) — Bone biopsy (single location) Glutamate-receptor 46 y, male Subacute cerebellar degeneration Lymph node (submandibular) CUP (grade 3 tumor, not further specified) Lymph node excision Anti-Tr 72 y, male Encephalomyelitis Sigmoid, lymph nodes (mediastinum) — § Colonoscopy, (follow-up PET-CT) Ma-2 78 y, female Lambert-Eaton syndrome Lymph nodes (multifocal), bone (multifocal), lung — ‖ Lymph node biopsy Anti-calcium channel (N-type) 67 y, male Subacute sensory neuropathy Intestinal PET-focus Mesenteric lymphoma Endoscopic biopsy (negative), laparotomy (not tested) 48 y, male Limbic encephalitis Lung (no information available) (no information available) — 51 y, female Limbic encephalitis Lung (see Fig 1 ) SCLC Endosonography-guided biopsy Anti-NMDA receptor
ANNA1, antineuronal autoantibody 1 (synonym Anti-Hu); CUP, cancer of unknown primary; other autoantibodies named of the first two letters of the index patients; CV2/CRMP5, collapsin response mediator protein 5; GABA, gamma-aminobutyric acid; NMDA, N-methyl D-aspartate; NSCLC, non–small cell lung cancer; SCLC, small cell lung cancer.
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Discussion
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Limitations
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Conclusions
Get Radiology Tree app to read full this article<
Acknowledgments
Get Radiology Tree app to read full this article<
Supplementary Data
Get Radiology Tree app to read full this article<
Appendix S1
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
References
1. Stich O., Rauer S.: Paraneoplastic neurological syndromes. Nervenarzt 2013; 84: pp. 455-460.
2. Rauer S.: Paraneoplastic neurological syndromes. Fortschr Neurol Psychiatr 2011; 79: pp. 51-58. quiz 59-61
3. Toothaker T.B., Rubin M.: Paraneoplastic neurological syndromes: a review. Neurologist 2009; 15: pp. 21-33.
4. Graus F., Dalmau J.: Paraneoplastic neurological syndromes. Curr Opin Neurol 2012; 25: pp. 795-801.
5. Graus F., Delattre J.Y., Antoine J.C., et. al.: Recommended diagnostic criteria for paraneoplastic neurological syndromes. J Neurol Neurosurg Psychiatry 2004; 75: pp. 1135-1140.
6. Titulaer M.J., Soffietti R., Dalmau J., et. al.: Screening for tumours in paraneoplastic syndromes: report of an EFNS task force. Eur J Neurol 2011; 18: 19–e3
7. Sioka C., Fotopoulos A., Kyritsis A.P.: Paraneoplastic neurological syndromes and the role of PET imaging. Oncology 2010; 78: pp. 150-156.
8. Sheikhbahaei S., Marcus C., Fragomeni R.S., et. al.: Whole body FDG-PET and FDG-PET/CT in patients with suspected paraneoplastic syndrome: a systematic review and meta-analysis of diagnostic accuracy. J Nucl Med 2016;
9. Moher D., Liberati A., Tetzlaff J., et. al.: Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6: pp. e1000097.
10. Liberati A., Altman D.G., Tetzlaff J., et. al.: The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 2009; 6: pp. e1000100.
11. Schramm N., Rominger A., Schmidt C., et. al.: Detection of underlying malignancy in patients with paraneoplastic neurological syndromes: comparison of 18F-FDG PET/CT and contrast-enhanced CT. Eur J Nucl Med Mol Imaging 2013; 40: pp. 1014-1024.
12. Vaidyanathan S., Pennington C., Ng C.Y., et. al.: 18F-FDG PET-CT in the evaluation of paraneoplastic syndromes: experience at a regional oncology centre. Nucl Med Commun 2012; 33: pp. 872-880.
13. Matsuhisa A., Toriihara A., Kubota K., et. al.: Utility of F-18 FDG PET/CT in screening for paraneoplastic neurological syndromes. Clin Nucl Med 2012; 37: pp. 39-43.
14. Selva-O’Callaghan A., Grau J.M., Gamez-Cenzano C., et. al.: Conventional cancer screening versus PET/CT in dermatomyositis/polymyositis. Am J Med 2010; 123: pp. 558-562.
15. McKeon A., Apiwattanakul M., Lachance D.H., et. al.: Positron emission tomography-computed tomography in paraneoplastic neurologic disorders: systematic analysis and review. Arch Neurol 2010; 67: pp. 322-329.
16. Bannas P., Weber C., Derlin T., et. al.: 18F-FDG-PET/CT in the diagnosis of paraneoplastic neurological syndromes: a retrospective analysis. Eur Radiol 2010; 20: pp. 923-930.
17. Hadjivassiliou M., Alder S.J., Van Beek E.J., et. al.: PET scan in clinically suspected paraneoplastic neurological syndromes: a 6-year prospective study in a regional neuroscience unit. Acta Neurol Scand 2009; 119: pp. 186-193.
18. Patel R.R., Subramaniam R.M., Mandrekar J.N., et. al.: Occult malignancy in patients with suspected paraneoplastic neurologic syndromes: value of positron emission tomography in diagnosis. Mayo Clin Proc 2008; 83: pp. 917-922.
19. Younes-Mhenni S., Janier M.F., Cinotti L., et. al.: FDG-PET improves tumour detection in patients with paraneoplastic neurological syndromes. Brain 2004; 127: pp. 2331-2338.
20. Berner U., Menzel C., Rinne D., et. al.: Paraneoplastic syndromes: detection of malignant tumors using [(18)F]FDG-PET. Q J Nucl Med 2003; 47: pp. 85-89.
21. Rees J.H., Hain S.F., Johnson M.R., et. al.: The role of [18F]fluoro-2-deoxyglucose-PET scanning in the diagnosis of paraneoplastic neurological disorders. Brain 2001; 124: pp. 2223-2231.
22. Kristensen S.B., Hess S., Petersen H., et. al.: Clinical value of FDG-PET/CT in suspected paraneoplastic syndromes: a retrospective analysis of 137 patients. Eur J Nucl Med Mol Imaging 2015; 42: pp. 2056-2063.
23. Vatankulu B., Yilmaz Aksoy S., Asa S., et. al.: Accuracy of FDG-PET/CT and paraneoplastic antibodies in diagnosing cancer in paraneoplastic neurological syndromes. Rev Esp Med Nucl Imagen Mol 2016; 35: pp. 17-21.
24. Pena Pardo F.J., Garcia Vicente A.M., Amo-Salas M., et. al.: Utility of 18F-FDG-PET/CT in patients suspected of paraneoplastic neurological syndrome: importance of risk classification. Clin Transl Oncol 2017;
25. Bossuyt P.M., Reitsma J.B., Bruns D.E., et. al.: The STARD statement for reporting studies of diagnostic accuracy: explanation and elaboration. Ann Intern Med 2003; 138: pp. W1-W12.
26. Bossuyt P.M., Reitsma J.B., Bruns D.E., et. al.: STARD 2015: an updated list of essential items for reporting diagnostic accuracy studies. Radiology 2015; 277: pp. 826-832.
27. Giometto B., Grisold W., Vitaliani R., et. al.: Paraneoplastic neurologic syndrome in the PNS Euronetwork database: a European study from 20 centers. Arch Neurol 2010; 67: pp. 330-335.
28. Raspotnig M., Vedeler C., Storstein A.: Paraneoplastic neurological syndromes in lung cancer patients with or without onconeural antibodies. J Neurol Sci 2015; 348: pp. 41-45.
29. Zer A., Domachevsky L., Rapson Y., et. al.: The role of 18F-FDG PET/CT on staging and prognosis in patients with small cell lung cancer. Eur Radiol 2016; 26: pp. 3155-3161.
30. Escalona S., Blasco J.A., Reza M.M., et. al.: A systematic review of FDG-PET in breast cancer. Med Oncol 2010; 27: pp. 114-129.
31. Maxim L.D., Niebo R., Utell M.J.: Screening tests: a review with examples. Inhal Toxicol 2014; 26: pp. 811-828.
32. von Schulthess G.K., Steinert H.C., Hany T.F.: Integrated PET/CT: current applications and future directions. Radiology 2006; 238: pp. 405-422.
33. Kwee T.C., Basu S., Cheng G., et. al.: FDG PET/CT in carcinoma of unknown primary. Eur J Nucl Med Mol Imaging 2010; 37: pp. 635-644.
34. Facey K., Granados A., Guyatt G., et. al.: Generating health technology assessment evidence for rare diseases. Int J Technol Assess Health Care 2014; 30: pp. 416-422.