Rationale and Objectives
Hybrid single photon-emission computed tomographic (SPECT) and computed tomographic (CT) imaging for the investigation of neuroendocrine tumors allows the fusion of functional and anatomic information in a rapid and efficient method. The aim of this study was to assess the incremental diagnostic value of 111 In pentetreotide SPECT/CT imaging compared with traditional planar and SPECT imaging with respect to lesion localization and characterization and reader confidence.
Materials and Methods
Forty-nine patients (23 male, 26 female; mean age, 56.9 years; range, 14–88 years) who underwent 111 In pentetreotide planar, SPECT, and SPECT/CT imaging were eligible for this retrospective study, including patients with suspected or confirmed carcinoid tumors ( n = 24), endocrine pancreatic tumors ( n = 18), medullary thyroid cancer ( n = 3), paragangliomas ( n = 2), and multiple endocrine neoplasia type I ( n = 2). Planar and SPECT images were reviewed by two blinded readers, followed by interpretation using additional SPECT/CT images in a subsequent session. A third reader provided consensus in cases with disagreements.
Results
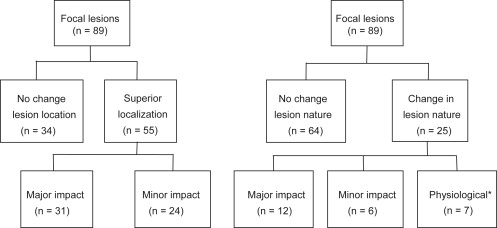
In 55 of 89 lesions (61.8%), 111 In pentetreotide SPECT/CT imaging improved lesion localization compared to planar and SPECT imaging; in 25 of 89 lesions (28.1%), SPECT/CT imaging changed lesion classification. In 20 of 49 patients (40.8%) for reader 1 and 14 of 49 patients (28.6%) for reader 2, 111 In pentetreotide SPECT/CT imaging provided incremental diagnostic value, which was considered likely to affect patient management in twelve of 20 and seven of 14 patients, respectively. Increased reader confidence was found in 32 of 49 patients (65.3%) for both readers with uniformly high confidence after SPECT/CT interpretation.
Conclusions
Hybrid 111 In pentetreotide SPECT/CT imaging provides incremental diagnostic value and greater reader confidence over planar and SPECT imaging. This is achieved though superior lesion localization, the identification of physiologic activity, and additional anatomic information derived from the nondiagnostic CT portion of the study.
Since its introduction almost two decades ago, somatostatin receptor scintigraphy has become the imaging modality of choice for the evaluation of neuroendocrine tumors (NETs), taking advantage of the overexpression of somatostatin receptors at the cell membrane by this group of neoplasms, to allow imaging with radiolabeled peptide somatostatin analogues . Hybrid single photon-emission computed tomographic (SPECT) and computed tomographic (CT) imaging, also referred to as transmission emission tomography or functional anatomic mapping, is a novel technology that combines functional and structural information in a rapid and efficient method, using integrated gamma cameras with inline CT scanners.
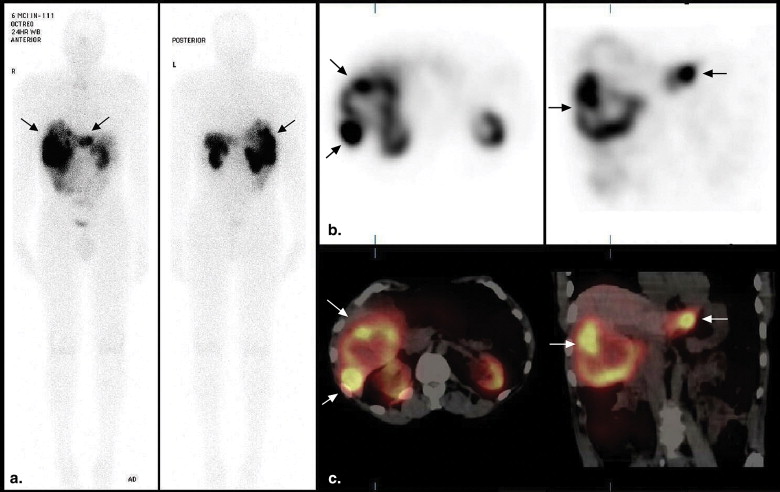
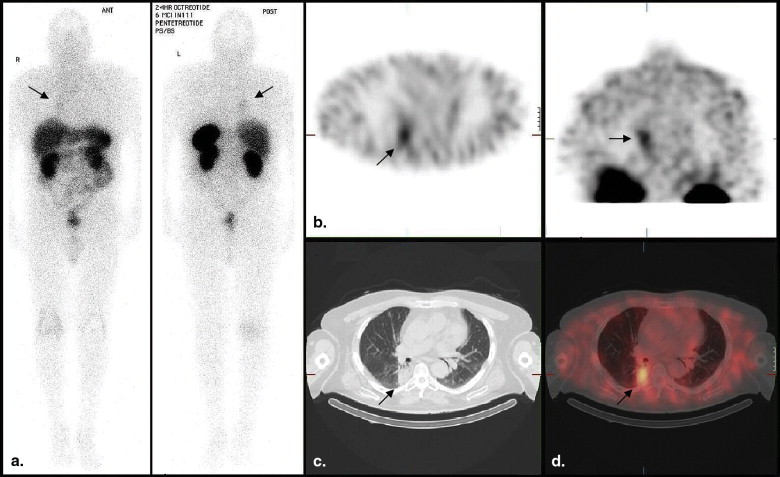
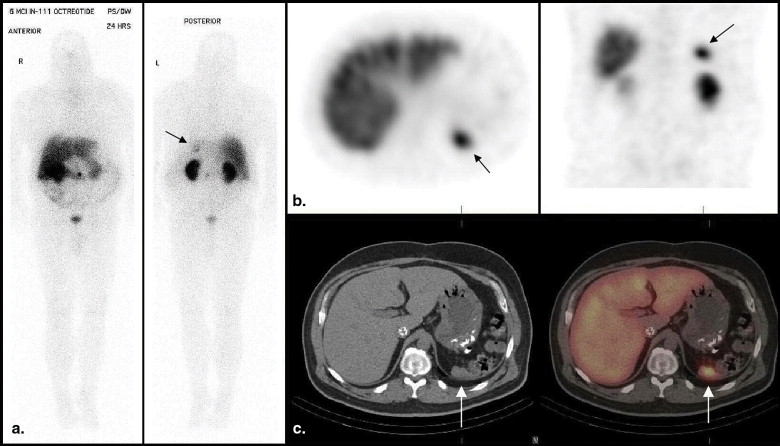
A recent review found evidence for superiority of SPECT/CT imaging over the current standard (planar and SPECT) imaging in bone, somatostatin receptor, parathyroid, and adrenal scintigraphy, although it was commented that there are limited clinical studies at present . The improvement in the diagnostic performance of somatostatin receptor scintigraphic (SRS) SPECT/CT imaging derives from superior anatomic localization of activity (and therefore lesion characterization) and the application of a CT algorithm to correct for photon attenuation in SPECT images . The CT component is usually nondiagnostic in quality (reduced tube current), without contrast enhancement, although the CT images may still provide useful structural information. The additional radiation exposure from the low-dose CT imaging is approximated at 2 to 4 mSv, depending on the field of view . Image acquisition of both components occurs with the patient in the same bed position. Coregistration of both tomographic sets is immediate and more precise (because of reduced patient movement) compared to software methods of image coregistration or side-by-side viewing of functional and structural information.
Get Radiology Tree app to read full this article<
Materials and methods
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Results
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Table 1
Incremental Diagnostic Value of SPECT/CT Imaging, Impact on Patient Management, and Anatomic Findings
Change in Management Patient Diagnosis SPECT/CT Region Reader 1 Reader 2 Incremental Value of SPECT/CT Imaging Anatomic Findings on CT Imaging 2 Paraganglioma Abdomen, Thorax Minor ∗ Major † R1 mesenteric Dx → para-aortic Dx
R2 bowel N → para-aortic Dx Lung nodules, pancreatic mass, para-aortic LN 5 Glucagonoma Abdomen None ‡ Major R2 liver Dx → gallbladder N
R2 bowel Dx → splenule Gallbladder, splenule 9 Gastrinoma Abdomen Minor Minor R1, R2 pancreatic Dx → mesenteric Dx Splenule, mesenteric LN, bowel N 10 EPT Abdomen Major Major R1 diagnosed pancreatic and gastric Dx R2 mesenteric Dx → pancreatic and gastric Dx Gastric wall thickening, pancreatic mass 11 Paraganglioma Abdomen, Thorax Minor Minor R1, R2 superior localization of multiple foci Multiple bone lesions (humeri, ribs, thoracic spine, pelvic)
Liver lesion 13 Gastrinoma Abdomen Major Major R1, R2 liver Dx → duodenum N Duodenum N 14 Carcinoid Abdomen Minor None R1 mesenteric Dx → small-bowel Dx Small-bowel mass 15 Carcinoid Abdomen None Major R2 suprapubic Dx → bowel N Suprapubic bowel N 16 EPT Abdomen, Thorax Minor Minor R1, R2 thyroid N Mediastinal and para-aortic LN, thyroid N 18 NET Head and neck
Abdomen Minor Minor R1 diagnosed maxillary sinus Dx
Auricular Dx → mastoid airspace Dx Diagnosed mediastinal Dx
R2 diagnosed maxillary sinus Dx
Diagnosed mastoid airspace Dx
Supraclavicular muscle N → mediastinal Dx Mastoid airspace opacification
Maxillary sinus opacification, mediastinal adenopathy 20 Carcinoid Abdomen Major Major R1 diagnosed liver and mesenteric Dx
R2 diagnosed liver Dx Liver lesion, mesenteric mass 21 MTC Abdomen, Thorax Major Minor R1 diagnosed humeral Dx
R2 localization of humeral Dx Humeral bone lesion, thyroid N 22 Carcinoid Abdomen Major None R1 diagnosed liver Dx Liver lesion, pancreatic mass 23 MTC Abdomen Minor Major R1 localized thoracic Dx
R2 diagnosed thoracic Dx Right upper lobe lung infiltrate, probably in external-beam radiation field 33 EPT Abdomen None Minor R2 pancreatic Dx → portocaval Dx Portocaval LN 34 Carcinoid Abdomen Major None R1 diagnosed pancreatic Dx Pancreatic mass 35 Carcinoid Abdomen Major None R1 diagnosed adrenal Dx Adrenal mass 37 Carcinoid Abdomen, Thorax Major None R1 diagnosed adrenal Dx Adrenal mass, gastric wall thickening 38 Carcinoid Abdomen, Thorax Minor Minor R1, R2 abdominal Dx → pancreatic Dx Pancreatic mass 41 Carcinoid Abdomen Major None R1 diagnosed pelvic Dx Pelvic mass, liver lesions, pancreatic mass 42 MEN type I Abdomen Major None R1 diagnosed superficial skin contamination Abdominal wall skin N 43 Carcinoid Abdomen Major None R1 liver Dx → gallbladder N Gallbladder N 44 EPT Abdomen Major None R1 diagnosed pancreatic Dx Pancreatic mass
Dx, disease; EPT, endopancreatic tumor; LN, lymph node; MEN, multiple endocrine neoplasia; MTC, medullary thyroid carcinoma; N, normal (physiologic); NET, neuroendocrine tumor; R1, reader 1 interpretation; R2, reader 2 interpretation; SPECT, single photon-emission computed tomographic; CT, computed tomographic.
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Discussion
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Conclusion
Get Radiology Tree app to read full this article<
References
1. Krenning E.P., Kwekkeboom D.J., Bakker W.H., et. al.: Somatostatin receptor scintigraphy with [111In-DTPA-D-Phe1]- and [123I-Tyr3]-octreotide: the Rotterdam experience with more than 1000 patients. Eur J Nucl Med 1993; 20: pp. 716-731.
2. Rufini V., Calcagni M.L., Baum R.P.: Imaging of neuroendocrine tumors. Semin Nucl Med 2006; 36: pp. 228-247.
3. de Herder W.W., Kwekkeboom D.J., Valkema R., et. al.: Neuroendocrine tumors and somatostatin: imaging techniques. J Endocrinol Invest 2005; 28: pp. 132-136.
4. Buck A.K., Nekolla S., Ziegler S., et. al.: SPECT/CT. J Nucl Med 2008; 49: pp. 1305-1319.
5. Bybel B., Brunken R.C., DiFilippo F.P., et. al.: SPECT/CT imaging: clinical utility of an emerging technology. Radiographics 2008; 28: pp. 1097-1113.
6. Ruf J., Steffen I., Mehl S., et. al.: Influence of attenuation correction by integrated low-dose CT on somatostatin receptor SPECT. Nucl Med Commun 2007; 28: pp. 782-788.
7. Even-Sapir E., Keidar Z., Sachs J., et. al.: The new technology of combined transmission and emission tomography in evaluation of endocrine neoplasms. J Nucl Med 2001; 42: pp. 998-1004.
8. Moreira A.P., Duarte L.H., Vieira F., et. al.: Value of SPET/CT image fusion in the assessment of neuroendocrine tumours with 111In-pentetreotide scintigraphy. Rev Esp Med Nucl 2005; 24: pp. 14-18.
9. Perri M., Erba P., Volterrani D., et. al.: Octreo-SPECT/CT imaging for accurate detection and localization of suspected neuroendocrine tumors. Q J Nucl Med Mol Imaging 2008; 52: pp. 323-333.
10. Krausz Y., Keidar Z., Kogan I., et. al.: SPECT/CT hybrid imaging with 111In-pentetreotide in assessment of neuroendocrine tumours. Clin Endocrinol (Oxford) 2003; 59: pp. 565-573.
11. Pfannenberg A.C., Eschmann S.M., Horger M., et. al.: Benefit of anatomical-functional image fusion in the diagnostic work-up of neuroendocrine neoplasms. Eur J Nucl Med Mol Imaging 2003; 30: pp. 835-843.
12. Gabriel M., Hausler F., Bale R., et. al.: Image fusion analysis of (99 m)Tc-HYNIC-Tyr(3)-octreotide SPECT and diagnostic CT using an immobilisation device with external markers in patients with endocrine tumours. Eur J Nucl Med Mol Imaging 2005; 32: pp. 1440-1451.
13. Amthauer H., Denecke T., Rohlfing T., et. al.: Value of image fusion using single photon emission computed tomography with integrated low dose computed tomography in comparison with a retrospective voxel-based method in neuroendocrine tumours. Eur Radiol 2005; 15: pp. 1456-1462.
14. Hillel P.G., van Beek E.J.R., Taylor C., et. al.: The clinical impact of a combined gamma camera/CT imaging system on somatostatin receptor imaging of neuroendocrine tumours. Clin Radiol 2006; 61: pp. 579-587.
15. Ingui C.J., Shah N.P., Oates M.E.: Endocrine neoplasm scintigraphy: added value of fusing SPECT/CT images compared with traditional side-by-side analysis. Clin Nucl Med 2006; 31: pp. 665-672.