Rationale and Objectives
The aim of this study was to systematically review the quality of papers on the clinimetric properties of magnetic resonance imaging for the diagnosis of juvenile idiopathic arthritis in peripheral joints.
Materials and Methods
A review of Medline, EMBASE, the Database of Abstracts of Reviews of Effects, and the Cochrane Library was performed by using a systematic search strategy. Two independent reviewers evaluated selected articles by using Standards for Reporting of Diagnostic Accuracy (STARD) and Quality Assessment of Diagnostic Accuracy Studies (QUADAS) tools. Items were reported independently for STARD and QUADAS.
Results
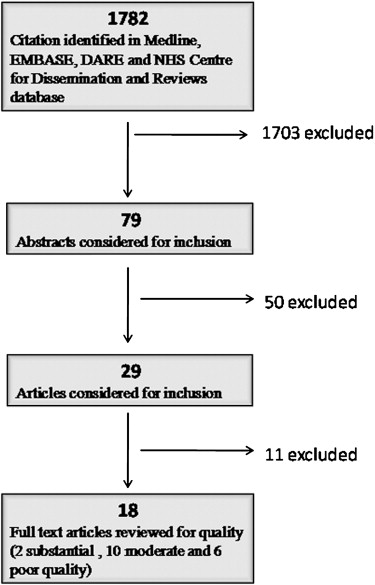
Eighteen studies (validity, n = 18; reliability, n = 3; responsiveness, n = 3) were included. Their overall quality of reporting of methods was fair. Methodological problems with the STARD system included a lack of reporting of exclusion criteria ( n = 14), partial or no information on operators’ expertise ( n = 14) or blinding ( n = 18), and deficient information on study time frames ( n = 12), treatments ( n = 10), or indeterminate results ( n = 18). The distribution of QUADAS scores was heterogeneous, with overall scores ranging between 3.5 (poor) and 16.5 (excellent) (maximum score, 17.5).
Conclusions
The quality of reporting of methods in studies on the magnetic resonance imaging assessment of juvenile idiopathic arthritis is heterogeneous and fair overall. Further methodological refinement of research design should be sought in future studies to provide stronger evidence for the value of novel techniques in clinical settings.
Juvenile idiopathic arthritis (JIA) is the most common rheumatic disease of childhood, with six to 19.6 incident cases per 100,000 children yearly in North America . Although the prognosis of this disease in children is generally favorable, with a majority of patients having no active synovitis in adulthood, persistent disease or progression of the disease is not uncommon, affecting children’s growth and development .
Laboratory indices of synovial inflammation are measures that are easily quantifiable but fail to provide accurate information about functional joint outcomes in JIA . Radiographs are usually nonspecific in the early stages of the disease, while magnetic resonance imaging (MRI) is a sensitive imaging tool for the detection of synovial hypertrophy and cartilage degeneration, which can be evaluated only indirectly by plain radiography .
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Material and methods
Data Sources and Search
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Inclusion Criteria
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Data Extraction and Outcome Measures
Get Radiology Tree app to read full this article<
Statistical Analysis
Get Radiology Tree app to read full this article<
Results
Search and Selection
Get Radiology Tree app to read full this article<
Table 1
Demographic Characteristics of Patients and Corresponding MRI Clinimetric Properties of the Selected Articles in This Review
Study Number of Patients, Number of Joints, Joint Types Mean (Range) Age (y) % Female Research Design Construct Validity Reliability Responsiveness Herve-Somma et al 24 (24 knees) 10 (3–18) 17 Retrospective Yes Eich et al 15 (11 knees, 4 hips) 6.3 (3.5–11.8) 7 Prospective Yes Yes Huppertz et al 21 (18 knees, 2 ankles, 1 elbow) 10.1 (1.4–18.9) 14 Prospective Yes Yes Murray et al 7 (14 hips) 11 (7–17) 4 Not stated Yes Remedios et al 11 (13 ankles) 9.7 (5–14) 9 Prospective Yes Ramsey et al 21 (21 knees) 13 (2–17) 11 Retrospective Uhl et al 21 (42 knees) 9.5 (5–13) 8 Not stated Yes Yes Cakmakci et al 38 (38 knees) 8 (2–17) 25 Prospective Yes Gylys-Morin et al 30 (30 knees) 10.2 (5–16) 21 Prospective Yes Yes El-Miedany et al 40 (40 knees) JIA patients, 40 control patients 11 (3–17) 32 Not stated Argyropoulou et al 28 (56 hips) 12.5 (2–24) 14 Not stated Yes Kight et al 18 (18 knees) JIA patients, 21 (21 knees) healthy children 8.1 (4.9–10.8) 39 Prospective Workie et al 13 (13 knees) 10.2 (6–16) 9 Prospective Yes Workie and Dardzinski 10 (10 wrists) 11.1 (5.2–15.7) 9 Prospective Yes Yes Graham et al 8 (8 knees) 11 (6–15) 7 Not stated Yes Gardner-Medwin 10 (10 knees) 9.4 (5.2–14.2) 7 Prospective Yes Nistala et al 34 (68 hips) 14.4 (4.3–19.7) No information Retrospective Workie et al 17 (17 knees) 10.3 (6.4–15.5) 13 Prospective Yes
JIA, juvenile idiopathic arthritis; MRI, magnetic resonance imaging.
Get Radiology Tree app to read full this article<
Qualitative Assessment of Quality of Reporting (STARD Tool)
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Participants (Items 3–6)
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Reference Standard (Item 7)
Get Radiology Tree app to read full this article<
Test Methods (Items 8–11)
Get Radiology Tree app to read full this article<
Statistics (Items 12 and 13)
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Time Frame of Study (Item 14)
Get Radiology Tree app to read full this article<
Characteristics of Participants (Item 15 and 16)
Get Radiology Tree app to read full this article<
Test Results (Items 17–20)
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Estimates (Items 21–24)
Get Radiology Tree app to read full this article<
Semiquantitative Assessment of Quality of Reporting (QUADAS Tool)
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Discussion
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Glossary
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Appendix A
Report of Quality in Reporting Using STARD Items for 18 Studies of JIA of the Peripheral Joints Using MRI
Get Radiology Tree app to read full this article<
Quality Study Response 1. Identify the article as a study of diagnostic accuracy Herve-Somma et al Yes (construct validity) Eich et al Yes (construct validity and responsiveness) Huppertz et al Yes (construct validity and responsiveness) Murray et al Yes (construct validity) Remedios et al Yes (construct validity) Ramsey et al Yes (criterion validity) Uhl et al Yes (construct validity) Cakmakci et al Yes (construct validity and responsiveness) Gylys-Morin et al Yes (construct validity and reliability); one case of cartilage thinning was confirmed at arthroscopy (criterion validity) El-Miedany et al Yes (construct validity) Argyropoulou et al Yes (construct validity) Kight et al Yes (construct validity) Workie et al Yes (construct validity) Workie and Dardzinski Yes (construct validity); parameters (Ktrans, Vp, Kep) of three models for arterial input function were evaluated Graham et al Yes (construct validity) and reliability Gardner-Medwin Yes (construct validity) Nistala et al Yes (construct validity and reliability) Workie et al Yes (construct validity) 2. State the research questions or study aims, such as estimating diagnostic accuracy or comparing accuracy between tests or across participant groups Herve-Somma et al To evaluate the role of contrast MRI in diagnosis, staging, and planning treatment of JRA Eich et al To evaluate the use of radiography, ultrasound, and MRI in assessment of affected knee and hips before and after intra-articular joint injection Huppertz et al To assess efficacy and potential toxicity of intra-articular corticosteroids therapy Murray et al To determine the value of contrast enhancement in MRI diagnosis of hip joint disease with JIA Remedios et al To compare clinical evaluation of hind foot synovitis with enhanced MRI in children with JIA Ramsey et al To compare clinical evaluation of hind foot synovitis with contrast-enhanced MR and evaluate the efficacy of intra-articular steroid injection Uhl et al To determine sensitivity and specificity of MR in diagnosis of JIA Cakmakci et al To establish correlation between clinical status and three-dimensional fast-spin contrast MRI in response to treatment Gylys-Morin et al To determine MRI findings in early JRA El-Miedany et al To assess the role of ultrasound vs MRI in inflammation in JIA of the knee Argyropoulou et al To establish the role of MRI in the assessment of hip joint involvement in clinical subtypes with JIA Kight et al To examine MRI T2 relaxation times in the weight-bearing cartilage of the distal femur in healthy children and children with JRA Workie et al Not reported Workie and Dardzinski Not reported Graham et al To assess feasibility of measuring synovial volume in the hand/wrist in polyarticular JIA Gardner-Medwin To evaluate if MRI of clinical unaffected joints is more sensitive than clinical assessment in identifying risk patients Nistala et al To assess diagnostic performance of clinical vs hip MRI and to determine clinical and serological predictors of MRI diagnosed hip arthritis Workie et al Utility of dynamic contrast-enhanced MRI based on pharmacokinetic modeling to evaluate disease activity in the knee and to correlate with clinical findings 3. The study population: inclusion and exclusion criteria, setting, and location where data were collected Herve-Somma et al Attending pediatric rheumatology clinic as outpatient; study population was well reported (age and gender); setting and location were not reported Eich et al EULAR criteria for JIA and one of the following: (1) failure of systemic treatment and physiotherapy at 3 mo, (2) local growth, (3) popliteal cyst; study population was well reported (age and gender); setting and location were not reported Huppertz et al Consent for intra-articular corticosteroids administration; population age and gender were reported; location was partially reported Murray et al Diagnose according to juvenile chronic arthritis criteria; age and gender reported; location not reported Remedios et al Age and gender reported; no inclusion criteria or location reported Ramsey et al Country hospital in Canada; included all patients referred with presumptive diagnosis of clinical monoarthritis; study population was well described (age, gender) Uhl et al Children included were followed for ≥1 y and diagnosed to have JIA of the knee by an experienced pediatric rheumatologist; age and gender reported; location of the study not included Cakmakci et al Patients attending pediatric rheumatology clinic as outpatients; study population (age, gender) included; location of the study was not reported Gylys-Morin et al Included according to ACR criteria, clinical evident arthritis in at least one knee, disease duration of <1 y; children were excluded if needed sedation for imaging or if injection of intra-articular steroids in the affected knee; age and gender reported; referred from rheumatology clinic El-Miedany et al Included according to ILAR criteria; excluded if injection of intra-articular steroids in the affected knee; study population (age and gender); location of the study reported Argyropoulou et al Included according to ILAR criteria; study population (age and sex) reported; location not reported Kight et al Included according to ACR criteria, active arthritis documented by rheumatologist in at least one examination, disease duration 2–7 y, girls, and age 4.9–10.8 y; excluded girls who needed sedation for imaging and if motion; location reported Workie et al Inclusion criteria: history of JRA; study population (age and gender) reported; location not reported Workie and Dardzinski Included children with history of JRA and good popliteal artery signal enhancement; study population (age and gender) reported; location not reported Graham et al Inclusion criteria: polyarticular disease, ACR criteria (at least three active joints, one hand); excluded children who needed sedation; study population (age and gender) and location of the study reported Gardner-Medwin Arthritis criteria for monoarthritis; Age and gender reported; tertiary center Nistala et al ILAR criteria; disease duration >6 mo; study (age and gender) reported; location not reported Workie et al ILAR criteria for active arthritis; study population (age and gender) reported; location not reported 4. Participant recruitment: was recruitment based on presenting symptoms, results from previous test, or the fact that the participants had received the index test or the reference standard? Herve-Somma et al Not reported Eich et al Presenting symptoms Huppertz et al Need for administration of intra-articular steroids Murray et al Not reported Remedios et al Presenting symptoms (pain and swelling) Ramsey et al Presenting symptoms Uhl et al Partial reported Cakmakci et al Not reported Gylys-Morin et al Presenting symptoms El-Miedany et al Presenting symptoms Argyropoulou et al Partial reported Kight et al Patients with JIA recruited by chart review and mail; controls were children of hospital personnel and healthy children who respond from an advertisement Workie et al History of JRA Workie and Dardzinski Not reported Graham et al Presenting symptoms Gardner-Medwin Presenting symptoms (monoarthritis) Nistala et al Presenting symptoms (hip pain) Workie et al Presenting symptoms 5. Participant sampling: was the study population a consecutive series of participants defined by selection criteria in items 3 and 4? Herve-Somma et al Not reported Eich et al Consecutive patients Huppertz et al Consecutive patients Murray et al Not reported Remedios et al Consecutive patients Ramsey et al Consecutive patients Uhl et al Not reported Cakmakci et al Consecutive patients Gylys-Morin et al Not mentioned but likely consecutive patients El-Miedany et al Consecutive patients Argyropoulou et al Consecutive patients Kight et al Not reported Workie et al Not reported Workie and Dardzinski Not reported Graham et al Not reported Gardner-Medwin Consecutive patients Nistala et al Partial (no information about consecutive patients) Workie et al Partial (no information about consecutive patients) 6. Data collection: was data collection planned before the index test and reference standard were performed (prospective study) or after (retrospective study)? Herve-Somma et al Retrospective Eich et al Prospective Huppertz et al Prospective Murray et al Not stated Remedios et al Prospective Ramsey et al Retrospective Uhl et al Not stated Cakmakci et al Not stated Gylys-Morin et al Prospective El-Miedany et al Prospective Argyropoulou et al Not stated Kight et al Prospective Workie et al Prospective Workie and Dardzinski Not stated Graham et al Prospective Gardner-Medwin Prospective Nistala et al Retrospective Workie et al Prospective 7. The reference standard and its rationale Herve-Somma et al Unenhanced MRI, test measure; enhanced MRI, reference standard; or x-ray, test measure; unenhanced and enhanced MRI, reference standards Eich et al MRI, US, and clinical findings, tests; follow-up, reference standard Huppertz et al For responsiveness: MRI and clinical assessment (tests), follow-up (reference standard); for construct validity: MRI (test), clinical assessment (reference standard) Murray et al Unenhanced MRI, test measure; enhanced MRI, reference standard Remedios et al MRI, test measure (scoring system); clinical findings, reference standard Ramsey et al Responsiveness of clinical findings to NSAIDs and intra-articular steroids: clinical response confirmed by MRI n = 1/4; accuracy of MRI: MRI diagnosis confirmed by arthroscopy n = 5/5; accuracy of x-rays compared by MRI false-negatives = 620; false-positives = 1/20 Uhl et al MRI, test measure; clinical findings, reference standard for discrimination of JIA vs non-JIA knees Cakmakci et al MRI and clinical findings, tests; follow-up, reference standard Gylys-Morin et al Evaluative role among internal items: MRI, test measure (40 items) and between MRI items and clinical synovitis; discriminative role: MRI for discrimination between JIA and control knees El-Miedany et al Evaluative role comparing unenhanced (test) vs enhanced MRI (reference standard): extent of pannus, joint effusion; evaluative role comparing x-rays, US, unenhanced MRI (tests) and enhanced MRI (reference standard): cartilage destruction; discriminative role: MRI vs US for discrimination between JIA and control subjects: 9 structural items Argyropoulou et al MRI, test measure (scoring system); clinical findings, reference standard Kight et al Discriminative role of MRI (test); clinical assessment (JIA vs healthy children): reference standard Workie et al Evaluative role of MRI, test measure (signal enhancement patterns) compared with clinical measures (CHAQ-Disability by parents and total knee scores [swelling, tenderness and limitation]): functional measure, reference standard, and evaluative role (internal relationship) of MRI components (enhancement rates of synovium vs femoral physis) Workie and Dardzinski Not applicable: assessment of differences/relationship between parameters of three models Graham et al MRI, test measure (total synovial volume); clinical scores, reference standard Gardner-Medwin Follow-up: reference standard Nistala et al MRI, outcome measure, reference standard; clinical and laboratory findings, predictor measures Workie et al MRI, test measure (scoring system); clinical findings, reference standard 8. Technical specifications of material and methods involved, including how and when measurements were taken, and/or cite references for index test and reference standard Herve-Somma et al Methods (MRI) well described Eich et al Methods (MRI) well described Huppertz et al Methods (MRI) well described Murray et al Methods (MRI) well described Remedios et al Methods (MRI) partially described Ramsey et al Methods (MRI) well described Uhl et al Methods (MRI) partially described Cakmakci et al Methods (MRI) well described Gylys-Morin et al Methods (MRI) well described El-Miedany et al Methods (MRI) well described Argyropoulou et al Methods (MRI) well described Kight et al Methods (MRI) well described Workie et al Methods (MRI) well described Workie and Dardzinski Methods (MRI) well described Graham et al Methods (MRI) well described Gardner-Medwin Methods (MRI) well described Nistala et al Methods (MRI) well described Workie et al Methods (MRI) well described 9. Definition of and rationale for the units, cutoffs, and/or categories of the results of the index tests and the reference standard Herve-Somma et al Not reported Eich et al Not applicable Huppertz et al Not applicable Murray et al Not reported Remedios et al Not reported Ramsey et al Not reported Uhl et al Not reported Cakmakci et al Not applicable Gylys-Morin et al Pettersson score (radiographs) El-Miedany et al Not reported Argyropoulou et al Not reported Kight et al Not reported Workie et al Not reported Workie and Dardzinski Not reported Graham et al Not reported Gardner-Medwin Not reported Nistala et al Global assessment of overall disease activity (VAS-PGA), CHAQ, and VAS global Workie et al Not reported 10. The number, training, and expertise of the persons executing and reading the index test and the reference standard Herve-Somma et al Independent observer review the MRI (no further information was given) Eich et al Not reported Huppertz et al Not reported Murray et al Two radiologists review all the images; no information about the clinicians; no details on reviewers expertise given Remedios et al Two radiologists review all the images; no information about the clinicians; no details on reviewers expertise given Ramsey et al Two pediatric radiologists with experience in musculoskeletal MRI, one blinded to clinical history; differences were review in consensus Uhl et al Five independent radiologists without knowledge of the clinical findings; no information about the clinicians Cakmakci et al An independent observer; no further information given Gylys-Morin et al Two radiologists blinded to clinical details read the MRI; in case of disagreement, a third radiologist provided independent interpretation; no further information given El-Miedany et al Not reported Argyropoulou et al One radiologist (specified) review blinded to clinical evaluation; no further information given Kight et al Operators involved in data analysis and image evaluation were blinded to study group; no further information given Workie et al Not reported Workie and Dardzinski Not reported Graham et al Rheumatologist for clinical inclusion, radiologist for MRI interpretation Gardner-Medwin MRI was reviewed by two consultant pediatric radiologist (expertise specified) Nistala et al Patients examined by a pediatric rheumatologist or an experienced pediatric rheumatologist in trainee (not otherwise specified); MRI review by two pediatric radiologist (names specified) Workie et al Not reported 11. Whether or not the readers of the index tests and reference standard were blind to the results of the other test and describe any other clinical information available to the readers Herve-Somma et al Not reported Eich et al Not reported Huppertz et al Not reported Murray et al Partial Remedios et al Not reported Ramsey et al Partial Uhl et al Partial Cakmakci et al Not reported Gylys-Morin et al Radiologist were blinded to details of the clinical data El-Miedany et al Not reported Argyropoulou et al One radiologist review blinded to clinical evaluation Kight et al Operators involved in data analysis and image evaluation were blinded to clinical information Workie et al Not reported Workie and Dardzinski Not reported Graham et al Rheumatologist were blinded to result of imaging; radiologists were blinded to the results of clinical assessment Gardner-Medwin Clinical examiners were blinded to MRI results Nistala et al Pediatric radiologists were blinded to clinical details Workie et al Not mentioned 12. Methods for calculating or comparing measures of diagnostic accuracy, and the statistical methods used to quantify uncertainty Herve-Somma et al Degree of cartilage destruction (MRI); χ 2 and Wilcoxon tests Eich et al Changes over time; no statistical methods reported Huppertz et al Not applicable Murray et al Visualization of pannus; Wilcoxon test Remedios et al Correlation between MRI signal and clinical outcome measures; no statistical methods reported Ramsey et al Clinical (MRI: reference standard; n = 4), x-rays (MRI: reference standard; n = 20), and MRI (arthroscopy and biopsy: reference standard; n = 5); no statistical methods reported Uhl et al Not mentioned, partial, presence or absence of JIA; terms sensitivity, specificity, positive and negative likelihood; ROC curves (no cutoff values reported); standard error Cakmakci et al Both MRI and clinical scores over time; Spearman correlation: association between MRI and clinical results Gylys-Morin et al Pettersson score (radiographs), MRI findings; t tests (continuous variables); nonparametric tests (ordinal variables); Spearman rank correlation: if at least one variable was ordinal; Pearson correlation: two continuous variables; ROC curves: nonparametric methods (Hanley and McNeil method): cut-offs reported only for x-rays (Pettersson scores), not for MRI El-Miedany et al US and MRI findings in affected and control subjects; no statistical methods reported Argyropoulou et al Clinical criteria for disease activity; test of normality of distribution; two-tailed t tests: differences in MRI grades between active and inactive patients. ANOVA: differences in MRI grades between patients with oligo, poly, and systemic forms Kight et al T2 values of JIA vs healthy knees; standard deviations for normalized distances; two-tailed t tests for analyses of differences in mean T2 relaxation times Workie et al Correlation between MRI signal and clinical outcome measures; Pearson correlation coefficient for parameter between distal femoral physis and synovium; unpaired t tests Workie and Dardzinski Poorly described; t tests for differences in signal enhancement using Ktrans, Kep, and Vp Graham et al Clinical measures (total hand swelling scores); Pearson correlation (parametric data) and Spearman correlation (nonparametric data) Gardner-Medwin Future clinical outcome; Fisher’s exact test and Mann-Whitney test Nistala et al MRI, core outcome variables; Pearson correlation: association between total MRI scores; Mann-Whitney test: comparison between hip MRI scores and clinician-defined active and inactive groups; χ 2 test: effect of damage on concordance between clinical and MRI scores Workie et al MRI parameters (Ktrans, Kep, Vp); Spearman’s rank correlation: between MRI and clinical/laboratory parameters; Wilcoxon test: comparison of results at different time points 13. Methods for calculating test reproducibility Herve-Somma et al Not applicable Eich et al Not done Huppertz et al Not applicable Murray et al Not done Remedios et al Not done Ramsey et al Not done Uhl et al Not done Cakmakci et al Kappa statistics: not appropriate for agreement between MRI and clinical results. Gylys-Morin et al κ (interobserver reliability): cutoff values for excellent, good, and marginal reliability El-Miedany et al Not applicable Argyropoulou et al Not done Kight et al Not done Workie et al Not applicable Workie and Dardzinski Not done Graham et al Interoperator reliability of synovial volume calculation; coefficient of variation (variation within and between observers) Gardner-Medwin Not done Nistala et al κ: intraobserver agreement; not appropriate for concordance between clinician’s assessment and MRI results Workie et al Not applicable
ACR, American College of Radiology; ANOVA, analysis of variance; CHAQ, Childhood Health Assessment Questionnaire; EULAR, European League Against Rheumatism; ILAR, International League of Associations for Rheumatology; JIA, juvenile idiopathic arthritis; JRA, juvenile rheumatoid arthritis; MRI, magnetic resonance imaging; NSAID, nonsteroidal anti-inflammatory drug; PGA, patient global assessment; ROC, receiver-operating characteristic; STARD, Standards for Reporting of Diagnostic Accuracy; VAS, visual analog scale.
Get Radiology Tree app to read full this article<
Appendix B
Report of the QUADAS Criteria of 18 Articles on Assessment of JIA of Peripheral Joints Using MRI
Get Radiology Tree app to read full this article<
Item Herve-Somma et al Eich et al Huppertz et al Murray et al Remedios et al Ramsey et al Uhl et al Cakmakci et al Gylys-Morin et al El-Miedany et al Argyropoulou et al Kight et al Workie et al Workie and Dardzinski Graham et al Gardner-Medwin Nistala et al Workie et al 1. Was the spectrum of patients representative of the patients in who will receive the test in practice? U/C U/C Yes No U/C U/C No U/C U/C U/C U/C U/C U/C No U/C U/C U/C U/C 2. Were selection criteria clearly described? U/C Yes Yes U/C No Yes No U/C Yes U/C No Yes No No Yes No Yes Yes 3. Is the reference standard likely to correctly classify the target condition? Yes Yes No Yes Yes Yes Yes Yes Yes Yes No Yes No N/A Yes Yes Yes Yes 4. Is the time period between reference standard and index test short enough to be reasonably sure that the target condition did not change between the two tests? U/C U/C Yes U/C No Yes N/A U/C U/C No No N/A N/A N/A Yes U/C Yes No 5. Did the whole sample or a random selection of the sample, receive verification using a reference standard of diagnosis? Yes Yes Yes Yes Yes Yes No Yes Yes U/C No No No No No Yes Yes Yes 6. Did patients receive the same reference standard regardless of the index test result? Yes Yes Yes Yes Yes Yes Yes Yes Yes Yes Yes Yes N/A N/A Yes Yes Yes Yes 7. Was the reference standard independent of the index test? No No N/A Yes Yes Yes Yes No Yes No U/C Yes No N/A Yes Yes Yes Yes 8. Was the execution of the index test described in sufficient detail to permit replication of the test? Yes Yes Yes Yes No No U/C Yes Yes U/C Yes Yes Yes Yes Yes Yes Yes Yes 9. Was the execution of the reference standard described in sufficient detail to permit replication of the test? Yes Yes N/A Yes U/C Yes No Yes Yes Yes Yes N/A N/A N/A Yes Yes Yes Yes 10. Were the index test results interpreted without knowledge of the results of the reference standard? No No N/A Yes U/C U/C Yes No Yes No No Yes No N/A U/C Yes U/C U/C 11. Were the index reference standard results interpreted without knowledge of the results of the index test? No No N/A No No U/C U/C No Yes No No Yes No No Yes Yes Yes No 12. Were the same clinical data available when test results were interpreted as would be available when the test is used in practice? Yes Yes Yes Yes Yes Yes Yes Yes Yes Yes U/C Yes Yes No Yes Yes Yes Yes 13. Were uninterpretable test results reported? No U/C No No No No No No No No No Yes No No No No No No 14. Were withdrawals from the study explained? N/A Yes U/C No No No No No N/A No No Yes No No No No Yes No
JIA, juvenile idiopathic arthritis; MRI, magnetic resonance imaging; N/A, not available; QUADAS, Quality Assessment of Studies of Diagnostic Accuracy Included in Systematic Reviews; U/C, unclear.
Items were rated “yes” if adequately described, “no” if not adequately described, “unclear” if the information in the article was unclear, and “not available” if no information was given in the article.
Get Radiology Tree app to read full this article<
References
1. Cassidy J.T., Petty R.E.: Juvenile rheumatoid arthritis.Textbook of pediatric rheumatology.1995.SaundersPhiladelphia, PA:pp. 135.
2. Oen K.: Long-term outcomes and predictors of outcomes for patients with juvenile idiopathic arthritis. Best Pract Res Clin Rheumatol 2002; 16: pp. 347-360.
3. Oen K., Malleson P.N., Cabral D.A., et. al.: Disease course and outcome of juvenile rheumatoid arthritis in a multicenter cohort. J Rheumatol 2002; 29: pp. 1989-1999.
4. Pispati Prakash K.: Evidence-based practice in rheumatology. APLAR J Rheumatol 2003; 6: pp. 44-49.
5. Graham T.B.: Imaging in juvenile arthritis. Curr Opin Rheumatol 2005; 17: pp. 574-578.
6. Roposch A., Moreau N.M., Uleryk E., Doria A.S.: Developmental dysplasia of the hip: quality of reporting of diagnostic accuracy for US. Radiology 2006; 241: pp. 854-860.
7. Lijmer J.G., Mol B.W., Heisterkamp S., et. al.: Empirical evidence of design-related bias in studies of diagnostic tests. JAMA 1999; 282: pp. 1061-1066.
8. Deville W.L., Bezemer P.D., Bouter L.M.: Publications on diagnostic test evaluation in family medicine journals: an optimal search strategy. J Clin Epidemiol 2000; 53: pp. 65-69.
9. Smidt N., Rutjes A.W., van der Windt D.A., et. al.: Quality of reporting of diagnostic accuracy studies. Radiology 2005; 235: pp. 347-353.
10. Reid M.C., Lachs M.S., Feinstein A.R.: Use of methodological standards in diagnostic test research. Getting better but still not good. JAMA 1995; 274: pp. 645-651.
11. Bossuyt P.M., Reitsma J.B., Bruns D.E., et. al.: Towards complete and accurate reporting of studies of diagnostic accuracy: the STARD initiative. Clin Chem 2003; 49: pp. 1-6.
12. Whiting P., Rutjes A.W., Reitsma J.B., et. al.: The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol 2003; 3: pp. 25.
13. Altman D.G.: Practical statistics for medical research.1991.Chapman & HallLondon
14. Fleiss J.L.: Statistical methods for rates and proportions.1981.John WileyNew York
15. Tynjala P., Honkanen V., Lahdenne P.: Intra-articular steroids in radiologically confirmed tarsal and hip synovitis in juvenile idiopathic arthritis. Clin Exp Rheumatol 2004; 22: pp. 643-648.
16. Johnson K., Wittkop B., Haigh F., et. al.: The early magnetic resonance imaging features of the knee in juvenile idiopathic arthritis. Clin Radiol 2002; 57: pp. 466-471.
17. Yulish B.S., Lieberman J.M., Newman A.J., et. al.: Juvenile rheumatoid arthritis: assessment with MR imaging. Radiology 1987; 165: pp. 149-152.
18. Senac M.O., Deutsch D., Bernstein B.H., et. al.: MR imaging in juvenile rheumatoid arthritis. AJR Am J Roentgenol 1988; 150: pp. 873-878.
19. Adib N., Silman A., Thomson W.: Outcome following onset of juvenile idiopathic inflammatory arthritis: I. frequency of different outcomes. Rheumatology (Oxford) 2005; 44: pp. 995-1001.
20. Adib N., Silman A., Thomson W.: Outcome following onset of juvenile idiopathic inflammatory arthritis: II. predictors of outcome in juvenile arthritis. Rheumatology (Oxford) 2005; 44: pp. 1002-1007.
21. Kuseler A., Pedersen T.K., Gelineck J., Herlin T.: A 2 year followup study of enhanced magnetic resonance imaging and clinical examination of the temporomandibular joint in children with juvenile idiopathic arthritis. J Rheumatol 2005; 32: pp. 162-169.
22. Arabshahi B., Cron R.Q.: Temporomandibular joint arthritis in juvenile idiopathic arthritis: the forgotten joint. Curr Opin Rheumatol 2006; 18: pp. 490-495.
23. Scolozzi P., Bosson G., Jaques B.: Severe isolated temporomandibular joint involvement in juvenile idiopathic arthritis. J Oral Maxillofac Surg 2005; 63: pp. 1368-1371.
24. Neidel J., Boehnke M., Kuster R.M.: The efficacy and safety of intraarticular corticosteroid therapy for coxitis in juvenile rheumatoid arthritis. Arthritis Rheum 2002; 46: pp. 1620-1628.
25. Uhl M., Krauss M., Kern S., et. al.: The knee joint in early juvenile idiopathic arthritis. An ROC study for evaluating the diagnostic accuracy of contrast-enhanced MR imaging. Acta Radiologica 2001; 42: pp. 6-9.
26. Gardner-Medwin J.M., Killeen O.G., Ryder C.A.J., et. al.: Magnetic resonance imaging identifies features in clinically unaffected knees predicting extension of arthritis in children with monoarthritis. J Rheumatol 2006; 33: pp. 2337-2343.
27. Nistala K., Babar J., Johnson K., et. al.: Clinical assessment and core outcome variables are poor predictors of hip arthritis diagnosed by MRI in juvenile idiopathic arthritis. Rheumatology (Oxford) 2007; 46: pp. 699-702.
28. Gylys-Morin V.M., Graham T.B., Blebea J.S., et. al.: Knee in early juvenile rheumatoid arthritis: MR imaging findings. Radiology 2001; 220: pp. 696-706.
29. Ramsey S.E., Cairns R.A., Cabral D.A., et. al.: Knee magnetic resonance imaging in childhood chronic monarthritis. J Rheumatol 1999; 26: pp. 2238-2243.
30. Argyropoulou M.I., Fanis S.L., Xenakis T., et. al.: The role of MRI in the evaluation of hip joint disease in clinical subtypes of juvenile idiopathic arthritis. Br J Radiol 2002; 75: pp. 229-233.
31. Herve-Somma C.M., Sebag G.H., Prieur A.M., et. al.: Juvenile rheumatoid arthritis of the knee: MR evaluation with Gd-DOTA. Radiology 1992; 182: pp. 93-98.
32. Murray J.G., Ridley N.T.F., Mitchell N., Rooney M.: Juvenile chronic arthritis of the hip: Value of contrast-enhanced MR imaging. Clin Radiol 1996; 51: pp. 99-102.
33. Remedios D., Martin K., Kaplan G., et. al.: Juvenile chronic arthritis: diagnosis and management of tibio-talar and sub-talar disease. Br J Rheumatol 1997; 36: pp. 1214-1217.
34. Cakmakci H., Kovanlikaya A., Unsal E.: Short-term follow-up of the juvenile rheumatoid knee with fat-saturated 3D MRI. Pediatr Radiol 2001; 31: pp. 189-195.
35. Kight A.C., Dardzinski B.J., Laor T., Graham T.B.: Magnetic resonance imaging evaluation of the effects of juvenile rheumatoid arthritis on distal femoral weight-bearing cartilage. Arthritis Rheum 2004; 50: pp. 901-905.
36. Graham T.B., Laor T., Dardzinski B.J.: Quantitative magnetic resonance imaging of the hands and wrists of children with juvenile rheumatoid arthritis. J Rheumatol 2005; 32: pp. 1811-1820.
37. Workie D.W., Dardzinski B.J.: Quantifying dynamic contrast-enhanced MRI of the knee in children with juvenile rheumatoid arthritis using an arterial input function (AIF) extracted from popliteal artery enhancement, and the effect of the choice of the AIF on the kinetic parameters. Magnc Reson Med 2005; 54: pp. 560-568.
38. Workie D.W., Dardzinski B.J., Graham T.B., et. al.: Quantification of dynamic contrast-enhanced MR imaging of the knee in children with juvenile rheumatoid arthritis based on pharmacokinetic modeling. Magn Reson Imaging 2004; 22: pp. 1201-1210.
39. Workie D.W., Graham T.B., Laor T., et. al.: Quantitative MR characterization of disease activity in the knee in children with juvenile idiopathic arthritis: a longitudinal pilot study. Pediatr Radiol 2007; 37: pp. 535-543.
40. Eich G.F., Halle F., Hodler J., et. al.: Juvenile chronic arthritis: imaging of the knees and hips before and after intraarticular steroid injection. Pediatr Radiol 1994; 24: pp. 558-563.
41. Huppertz H.I., Tschammler A., Horwitz A.E., Schwab K.O.: Intraarticular corticosteroids for chronic arthritis in children: efficacy and effects on cartilage and growth. J Pediatr 1995; 127: pp. 317-321.
42. El-Miedany Y.M., Housny I.H., Mansour H.M., et. al.: Ultrasound versus MRI in the evaluation of juvenile idiopathic arthritis of the knee. Joint Bone Spine 2001; 68: pp. 222-230.
43. Rosner B.: Fundamentals of biostatistics.1995.DuxburyBelmont, CA
44. Doria A.S., Babyn P.S., Feldman B.: A critical appraisal of radiographic scoring systems for assessment of juvenile idiopathic arthritis. Pediatr Radiol 2006; 36: pp. 759-772.
45. Ostergaard M., McQueen F., Bird P., et. al.: The OMERACT Magnetic Resonance Imaging Inflammatory Arthritis Group—advances and priorities. J Rheumatol 2007; 34: pp. 852-853.
46. Hanley J.A., McNeil B.J.: The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 1982; 143: pp. 29-36.
47. Moses L.E., Shapiro D., Littenberg B.: Combining independent studies of a diagnostic test into a summary ROC curve: data-analytic approaches and some additional considerations. Stat Med 1993; 12: pp. 1293-1316.
48. Wallace C.A., Ravelli A., Huang B., Giannini E.H.: Preliminary validation of clinical remission criteria using the OMERACT filter for select categories of juvenile idiopathic arthritis. J Rheumatol 2006; 33: pp. 789-795.
49. Singh G., Athreya B.H., Fries J.F., Goldsmith D.P.: Measurement of health status in children with juvenile rheumatoid arthritis. Arthritis Rheum 1994; 37: pp. 1761-1769.
50. Jaeschke R., Guyatt G., Sackett D.L.: Evidence-Based Medicine Working Group. Users’ guides to the medical literature. III. How to use an article about a diagnostic test. A. Are the results of the study valid?. JAMA 1994; 271: pp. 389-391.
51. Jaeschke R., Guyatt G.H., Sackett D.L.: Evidence-Based Medicine Working Group. Users’ guides to the medical literature. III. How to use an article about a diagnostic test. B. What are the results and will they help me in caring for my patients?. JAMA 1994; 271: pp. 703-707.
52. O’Dowd T.C.: Informing patients about clinical disagreement. Lancet 1989; 2: pp. 744.
53. Scott D.L., Coulton B.L., Popert A.J.: Long term progression of joint damage in rheumatoid arthritis. Ann Rheum Dis 1986; 45: pp. 373-378.
54. Sharp J.T.: An overview of radiographic analysis of joint damage in rheumatoid arthritis and its use in metaanalysis. J Rheumatol 2000; 27: pp. 254-260.
55. Rennie D.: Improving reports of studies of diagnostic tests: the STARD initiative. JAMA 2003; 289: pp. 89-90.
56. Feinstein A.R.: Clinimetric perspectives. J Chronic Dis 1987; 40: pp. 635-640.
57. Kirshner B., Guyatt G.: A methodological framework for assessing health indices. J Chronic Dis 1985; 38: pp. 27-36.
58. Nunnally J.: Psychometric theory.1978.McGraw-HillNew York
59. Patrick D.L., Erickson P.: Health status and health policy allocation resources to health care.1993.Oxford University PressNew York 198–202