Rationale and Objectives
The purpose of this study was to review the imaging findings associated with lobular carcinoma in situ (LCIS) of the breast with clinical and pathological correlation.
Materials and Methods
A database search of patients treated at our institution from 2002 to 2011 identified 26 patients with LCIS associated with an imaging abnormality that had imaging available for review. LCIS was diagnosed by core-needle or excision biopsy. Patients subsequently underwent excisional biopsy, mastectomy, or clinical follow-up. Patients’ mammography, ultrasonography (US), and magnetic resonance imaging (MRI) images were reviewed using the American College of Radiology Breast Imaging Reporting and Data System lexicon together with relevant clinical and pathology data.
Results
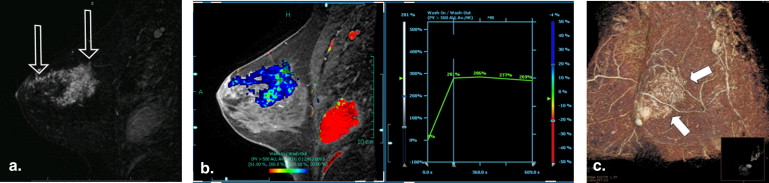
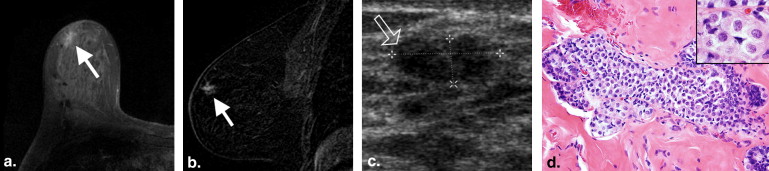
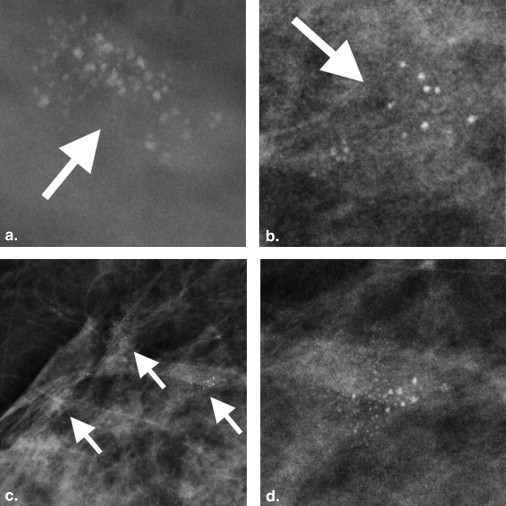
The 26 patients had 31 imaging lesions that yielded a histological diagnosis of LCIS by image-guided core-needle biopsy ( n = 29) or excision biopsy ( n = 2). Twenty-eight of 31 (90%) lesions yielding LCIS had a mammographic abnormality, 3/20 (15%) lesions had a US abnormality, and 6/7 (86%) had an abnormality on contrast-enhanced MRI. Calcifications were the most common mammographic finding, seen in 25/31 (80%) lesions. All three lesions seen on US were masses; the majority was irregular, hypoechoic, avascular, and had posterior shadowing. Non–mass-like enhancement was seen in five (71%) lesions with an MRI abnormality. Two (7%) patients developed subsequent malignancy at follow-up.
Conclusion
LCIS can have associated imaging abnormalities, most commonly grouped amorphous calcifications on mammography, a shadowing, avascular, irregular, hypoechoic mass on US, or heterogeneous non–mass-like enhancement with persistent enhancement kinetics on MRI.
Lobular carcinoma in situ (LCIS) is an uncommon, noninvasive lesion of the breast first described by Foote and Stewart in 1941. Histopathologically, it is a proliferation of atypical monotonous epithelial cells filling and distending the acinar units of a lobule. Depending on the appearance of these cells, LCIS can be categorized as classic or pleomorphic. LCIS is frequently multifocal and/or multicentric and often occurs bilaterally . Patients diagnosed with LCIS have an increased risk of invasive breast malignancy; Bodian et al reported an overall cumulative risk of breast cancer of 30%–35% in women who had had LCIS. It is generally accepted that both breasts are at increased risk of malignancy rather than only the breast where LCIS was detected , but not all studies show equal risk for both breasts . There is also some evidence of a precursor-product relationship between LCIS and invasive lobular carcinoma . LCIS has been described as clinically undetectable and with no known distinguishing radiologic features. It is often believed to be an incidental histopathological finding on image-guided or excisional biopsy that is performed for and targeted at a separate lesion . By retrospectively reviewing cases of LCIS directly associated with imaging findings which were the target for biopsy, we seek to better understand the spectrum of imaging findings that prompt image-guided biopsy and yield a diagnosis of LCIS. The histopathology and clinical outcomes of these patients were also documented.
Materials and methods
Patient Selection
Institutional review board approval was obtained for this study, which complied with the Health Insurance Portability and Accountability Act. We performed a database search of patients treated at our institution from January 1, 2002, to August 31, 2011, to identify all cases of LCIS associated with an imaging abnormality for which imaging findings were available for review. The single institution is a tertiary referral cancer center, where approximately 2000 patients are diagnosed with breast cancer each year. A total of 19,000 core-needle biopsies (CNB) were performed during the study period, 1994 of which yielded LCIS. Patients had to have been diagnosed with LCIS by image-guided CNB or excisional biopsy (EB), and LCIS had to be the highest grade lesion at the biopsy site. A total of 1963 of 1994 (98%) biopsies yielded LCIS as an incidental finding. We identified 26 patients with 31 LCIS lesions that met our inclusion criteria of LCIS associated with an imaging finding. Our institution’s electronic medical record was used to gather patients’ demographic and clinical information.
Imaging
Get Radiology Tree app to read full this article<
Mammography
Get Radiology Tree app to read full this article<
Sonography
Get Radiology Tree app to read full this article<
MRI
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Biopsies
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Histopathological Characteristics
Get Radiology Tree app to read full this article<
Results
Get Radiology Tree app to read full this article<
Mammographic Findings
Get Radiology Tree app to read full this article<
Table 1
Mammographic Findings of 31 LCIS Lesions
Mammographic Findings_n_ (%) ∗ Calcifications 25 (80) Morphology Amorphous 13 (42) Coarse heterogeneous 8 (26) Punctate 2 (6) Pleomorphic 1 (3) Dystrophic 1 (3) Distribution Diffuse 1 (3) Regional 2 (6) Segmental 2 (6) Group/cluster 20 (65) Architectural distortion 4 (13) Focal asymmetry 1 (3) No abnormality visualized 3 (10)
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Sonographic Findings
Get Radiology Tree app to read full this article<
MRI Findings
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Follow-up Findings
Get Radiology Tree app to read full this article<
Table 2
Clinical, Radiological, and Histopathological Features of 31 Lesions in 26 Patients with LCIS Found on Core Needle or Excisional Biopsy
Pt Age (y) R/L Size (cm) Imaging Modality CNB EB LCIS Grade Mx F/U (y) Subsequent Cancer MG US MRI Histology R/L Interval between LCIS and Cancer (y) Same Site as LCIS 1 49 R 5 AB N AB ND Benign 1–2 ND 2.1 — 2 ∗ 40 R 8 AB N AB Benign Benign 1 ND 4.9 — 3 62 L 0.4 AB ND ND Benign Benign 2–3 ND 2.5 — 4 46 L 10 N N AB Benign ND 1 PM 3.3 — 5 † 66 L 0.9 AB N ND Benign ND 1 ND 4.4 — 6 ‡ 62 L 1.3 AB ND ND Benign Benign 2–3 PM 6.1 — 62 L 0.5 AB ND ND Benign Benign 2 PM 6.1 — 62 L 0.3 AB ND ND Benign ND 2 PM 6.1 — 64 L 1.0 AB N ND Benign ND 2 PM 6.1 — 7 ‡ , § 40 L 2 AB N ND Benign ND 1 ND 9.1 — 42 L diffuse AB ND ND Benign ND 1 TM 7.1 IDC, DCIS L 6.9 Yes 8 52 R 7 AB AB ND Benign Benign 1–2 ND 4.9 — 9 62 R 0.8 AB ND ND Benign Benign 1 ND 1.5 — 62 L 1 N N AB Benign Benign 2–3 ND 1.5 — 10 60 R 2 AB AB ND Benign Benign 1–2 ND 1.3 — 11 50 R 2.5 AB N N Benign ND 1–2 ND 0.5 — 12 54 L 0.5 AB N ND Benign ND 1 ND 8.4 — 13 60 R 1.5 N AB AB Benign Benign 2 ND 5.3 — 14 57 L 4.3 AB N AB Benign Benign 1 ND 1.8 — 15 65 R 0.6 AB N ND Benign ND 1 ND 2 — 16 § 62 R 1 AB ND ND Benign Benign 1–2 ND 9.7 — 17 56 R 0.5 AB ND ND Benign ND 1 SM 9.4 ILC L 8.6 No SM 9.4 DCIS R 9.3 Yes 18 44 R 0.5 AB N ND Benign Benign 1 ND 1.5 — 19 55 L 6.5 AB N ND Benign Benign 2–3 ND 4.9 — 20 45 L 0.5 AB N ND ND Benign 1 ND 5.6 — 21 § 68 L 0.4 AB N ND Benign ND 1 ND 0.6 — 22 63 R 2 AB N ND Benign Benign 2–3 ND 0.5 — 23 60 R 0.3 AB N ND Benign ND 1 ND 2.5 — 24 59 L 2.8 AB ND ND Benign ND 1 ND 4.2 — 25 45 L 0.6 AB ND ND Benign Benign 2 ND 2.4 — 26 43 L 0.3 AB ND ND Benign ND 1 ND 4 —
AB, abnormal; B, bilateral; CNB, core needle biopsy; DCIS, ductal carcinoma in situ; EB, excisional biopsy; F/U, follow-up; IDC, invasive ductal carcinoma; ILC, invasive lobular carcinoma; L, left; LCIS, lobular carcinoma in situ; MG, mammogram; MRI, magnetic resonance imaging; Mx, mastectomy; N, normal; ND, not done; NA, not available; Pt, patient; PM, prophylactic mastectomy; R, right; SM, segmental mastectomy; TM, therapeutic mastectomy; US, ultrasound; y, years.
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Discussion
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
References
1. Foote F.J., Stewart F.: Lobular carcinoma in situ: a rare form of mammary carcinoma. Am J Pathol 1941; 17: pp. 491-496.
2. Anderson B.O., Calhoun K.E., Rosen E.L.: Evolving concepts in the management of lobular neoplasia. J Natl Compr Cancer Network 2006; 4: pp. 511-522.
3. Bodian C.A., Perzin K.H., Lattes R.: Lobular neoplasia: long term risk of breast cancer and relation to other risk factors. Cancer 1996; 78: pp. 1024-1034.
4. Silverstein M.J.: Ductal carcinoma in situ of the breast.1997.Williams & WilkinsBaltimore, MD 595–613
5. Page D.L., Simpson J.F.: What is atypical lobular hyperplasia and what does it mean for the patient?. J Clin Oncol 2005; 23: pp. 5432-5433.
6. Hwang E.S., Nyante S.J., Yi Chen Y., et. al.: Clonality of lobular carcinoma in situ and synchronous invasive lobular carcinoma. Cancer 2004; 100: pp. 2562-2572.
7. Pope T.L., Fechner R.E., Wilhelm M.C., et. al.: Lobular carcinoma in situ of the breast: mammographic features. Radiology 1988; 168: pp. 63-66.
8. Sonnenfeld M.R., Frenna T.H., Weidner N., et. al.: Lobular carcinoma in situ: mammographic-pathologic correlation of results of needle-directed biopsy. Radiology 1991; 181: pp. 363-367.
9. D’Orsi C.J., Mendelson E.B., Ikeda D.M., et. al.: Breast Imaging Reporting and Data System: ACR BI-RADS – Breast Imaging Atlas.2003.American College of RadiologyReston, VA
10. Krishnamurthy S., Bevers T., Kuerer H., et. al.: Multidisciplinary considerations in the management of high-risk breast lesions. AJR Am J Roentgenol 2012; 198: pp. W132-W140.
11. Beute B.J., Kalisher L., Hutter R.V.: Lobular carcinoma in situ of the breast: clinical, pathologic, and mammographic features. AJR Am J Roentgenol 1991; 157: pp. 257-265.
12. Liberman L., Sama M., Susnik B., et. al.: Lobular carcinoma in situ at percutaneous breast biopsy: surgical biopsy findings. AJR Am J Roentgenol 1999; 173: pp. 291-299.
13. Georgian-Smith D., Lawton T.J.: Calcifications of lobular carcinoma in situ of the breast: Radiologic-Pathologic Correlation. AJR Am J Roentgenol 2001; 176: pp. 1255-1259.
14. Middleton L.P., Grant S., Stephens T., et. al.: Lobular carcinoma in situ diagnosed by core needle biopsy: when should it be excised?. Mod Pathol 2003; 16: pp. 120-129.
15. Arpino G., Allred D.C., Mohsin S.K., et. al.: Lobular neoplasia on core-needle biopsy—clinical significance. Cancer 2004; 101: pp. 242-250.
16. Foster M.C., Helvie M.A., Gregory N.E., et. al.: Lobular carcinoma in situ or atypical lobular hyperplasia at core-needle biopsy: is excisional biopsy necessary?. Radiology 2004; 231: pp. 813-819.
17. Brenner R.J., Jackman R.J., Parker S.H., et. al.: Percutaneous core needle biopsy of radial scars of the breast: when is excision necessary?. AJR Am J Roentgenol 2002; 179: pp. 1179-1184.
18. Stein L.F., Zisman G., Rapelyea J.A., et. al.: Lobular carcinoma in situ of the breast presenting as a mass. AJR Am J Roentgenol 2005; 184: pp. 1799-1801.
19. Friedlander L.C., Roth S.O., Gavenonis S.C.: Results of MR imaging screening for breast cancer in high-risk patients with lobular carcinoma in situ. Radiology 2011; 261: pp. 421-427.
20. Sung J.S., Malak S.F., Bajaj P., et. al.: Screening breast MR imaging in women with a history of lobular carcinoma in situ. Radiology 2011; 261: pp. 414-420.
21. Destounis S.V., Murphy P.F., Seifert P.J., et. al.: Management of patients diagnosed with lobular carcinoma in situ at needle core biopsy at a community-based outpatient facility. AJR Am J Roentgenol 2012; 198: pp. 281-287.
22. Fisher E.R., Costantino J., Fisher B., et. al.: Pathologic findings from the National Surgical Adjuvant Breast Project (NSABP) Protocol B-17: five-year observations concerning lobular carcinoma in situ. Cancer 1996; 78: pp. 1403-1416.
23. O’Driscoll D., Britton P., Bobrow L., et. al.: Lobular carcinoma in situ on core biopsy – what is the clinical significance?. Clin Radiol 2001; 56: pp. 216-220.
24. Cohen M.A.: Cancer upgrades at excisional biopsy after diagnosis of atypical lobular hyperplasia or lobular carcinoma in situ at core-needle biopsy: some reasons why. Radiology 2004; 231: pp. 617-621.
25. Brem R.F., Lechner M.C., Jackman R.J., et. al.: Lobular neoplasia at percutaneous breast biopsy: variables associated with carcinoma at surgical excision. AJR Am J Roentgenol 2008; 190: pp. 637-641.
26. Berg W.A., Mrose H.E., Ioffe O.B.: Atypical lobular hyperplasia or lobular carcinoma in situ at core-needle breast biopsy. Radiology 2001; 218: pp. 503-509.
27. Sohn V.Y., Arthurs Z.M., Kim F.S., et. al.: Lobular neoplasia: is surgical excision warranted?. Am Surg 2008; 74: pp. 172-177.
![Figure 2, A 60-year-old woman with lobular carcinoma in situ (LCIS) presenting as calcifications and architectural distortion on screening mammography. Ultrasound-guided core-needle biopsy showed low-grade classic LCIS and radial scar. Subsequent excisional biopsy showed low- to intermediate-grade classic LCIS. (a) Magnification mammography shows architectural distortion with associated calcifications ( arrow ). (b) Mammogram after ultrasound-guided biopsy shows clip marker within area of distortion ( arrowhead ). Additional marker clip in lateral breast marks site of benign biopsy 6 years prior. (c) Photomicrograph illustrates lobular carcinoma in situ ( arrows ) adjacent to radial scar (hematoxylin and eosin [H & E] image ×20). Acinar spaces are filled and distended by neoplastic cells. (d) Photomicrograph illustrates radial scar ( arrow ) identified in surgical excision specimen (H & E image ×20). Note presence of central area of fibroelastosis bordered by dilated ducts demonstrating mild ductal epithelial hyperplasia without atypia.](https://storage.googleapis.com/dl.dentistrykey.com/clinical/LobularCarcinomaInSituoftheBreast/1_1s20S1076633212005405.jpg)